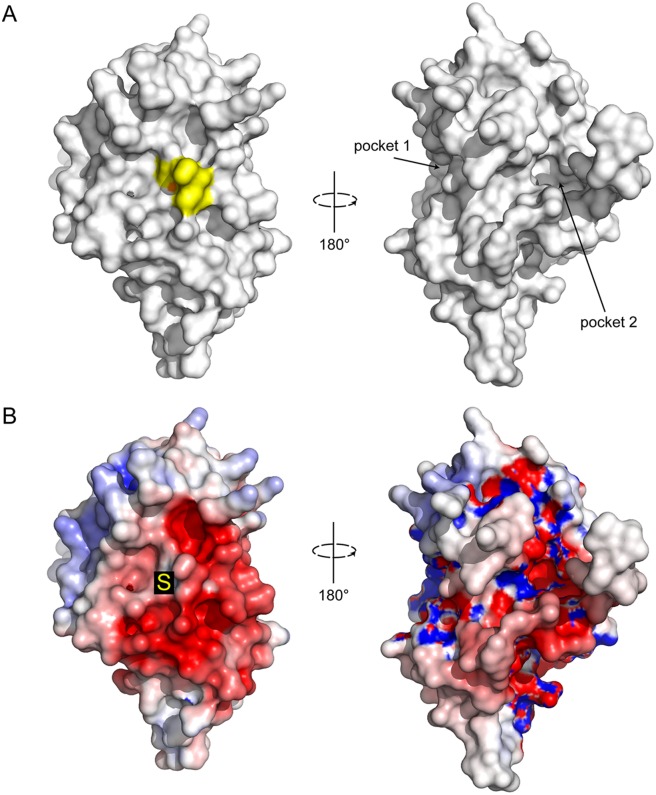
Fig 5. Surface properties of CtDsbA.
Surface representation for CtDsbA of the catalytic (left) and non-catalytic (right) faces. The active site residues Cys-Ser-Ala-Cys are colored yellow and the nucleophilic cysteine sulfur highlighted in orange. Pockets formed on the posterior face of the protein between H1 and H3 (pocket 1) and the N-terminal unstructured region and H6 (pocket 2) are labeled. B. Electrostatic surface representation of CtDsbA. Views are oriented as above. Electrostatic surface potential is contoured between -5 (red) and +5 (blue) kT/e. The nucleophilic cysteine is annotated with an S.