Abstract
Chronic stress causes deleterious changes in physiological function in systems ranging from neural cells in culture to laboratory rodents, sub-human primates and humans. It is notable, however, that the vast majority of research in this area has been conducted in males. In this review, we provide information about chronic stress effects on cognition in female rodents and contrast it with responses in male rodents. In general, females show cognitive resilience to chronic stressors which impair male cognitive function using spatial tasks including the radial arm maze, radial arm water maze, Morris water maze, Y-maze and object placement. Moreover, stress often enhances female performance in some of these cognitive tasks. Memory in females is not affected by stress in non-spatial memory tasks like recognition memory and temporal order recognition memory while males show impaired memory following stress. We discuss possible bases for these sex-dependent differences including the use of different strategies by the sexes to solve cognitive tasks. Whether the sex differences result from changes in non-mnemonic factors is also considered. Sex-dependent differences in alcohol and drug influences on stress responses are also described. Finally, the role of neurally derived estradiol in driving sex differences and providing resilience to stress in females is shown. The importance of determining the nature and extent of sex differences in stress responses is that such differences may provide vital information for understanding why some stress related diseases have different incidence rates between the sexes and for developing novel therapeutic treatments.
Keywords: Cognition, Resilience, Sex Differences, Spatial Memory, Stress
Introduction
Data has accumulated showing that stress, either acute or chronic, does not elicit identical responses in males and females. Sex differences range from physiological changes such as the amount of stress hormones released and weight lost, to differences in basic cellular responses and to changes in higher order neural responses such as learning and memory abilities (Luine and Gomez, 2015). Here, we focus on sex differences found in adult rodents after receiving chronic stresses. As in most neuroscience studies, females are not often investigated (Beery and Zucker, 2011), and thus, many conclusions are tentative and await confirmation in studies which consider stress effects in both sexes. However in general, females show resilience to chronic stressors which impair male cognitive function. We discuss possible bases for this pattern including use of different strategies by the sexes to solve cognitive tasks and how such differences may impact responses to stress. Whether sex differences result from stress-dependent changes in non-mnemonic factors which can influence performance on memory tasks or in processes that contribute to learning and memory are considered. Other factors including drugs and alcohol can also impact stress responses in a sex-dependent fashion. The role of both gonadal and neural derived estradiol in driving sex differences and providing resilience to stress in hippocampal-prefrontal cortex circuits in females is also discussed. Finally, these sexually differentiated responses are considered within the context that they could contribute to well-described sex differences in humans for coping with chronic stress and in the sexually divergent incidence of stress-related diseases. For example, women have a higher incidence of anxiety disorders, post-traumatic stress disorder (PTSD) and major depression than men while they have a lower incidence of alcohol and drug abuse than men (Bangasser and Valentino, 2014). Better understanding of sex differences in stress responding and the bases may inform the development of novel and more effective therapies for these disorders which are often precipitated by or related to stress and which pose an enormous burden on society.
2. Chronic stress elicits sexually differentiated effects on cognition
2.1. Learning and memory is impaired in males following chronic stress
In male rats and mice, chronic stress, as for example daily restraint or different daily stressors (unpredictable chronic stress, UCS), generally results in impaired learning and memory when tasks requiring the use of spatial information are tested (Conrad, 2010). Such tasks include the Morris water maze (MWM), eight-arm radial maze (RAM), object placement and the Y-maze. These tasks are dependent on an intact hippocampus and also require interaction with the prefrontal cortex (PFC) (Luine, 2015a). Our studies were the first to show that 21 days of restraint stress for 6 h/day impairs learning of male rats on the RAM (Luine et al, 1994)(See left two bars of Fig. 1A) and similar results were found on the MWM (Kitraki et al, 2004; McFadden et al, 2011). In tests assessing spatial memory (not learning), chronic stress also generally impairs male performance (See Conrad, 2010 for review). Spatial memory in both the object placement (Beck and Luine, 1999; Bowman et al, 2009; Gomez et al, 2012; Beck and Luine, 2002) and the Y-maze task is impaired following 3 weeks of daily restraint (Conrad et al, 2003; Wright and Conrad, 2005; Gomez et al, 2013). As shown for the object placement task (left two bars of Fig. 1B), unstressed, control males spend more time exploring the object in the new location (or a novel arm) rather than the old location or an old arm suggesting they remember old vs new locations or novel arms whereas stressed males spend the same amount of time exploring at both old and new suggesting poor memory. These impairments are time dependent and reversible because if stressed males are trained and tested on the RAM beginning at 18 days post stress, no impairments are present (Luine et al, 1996).
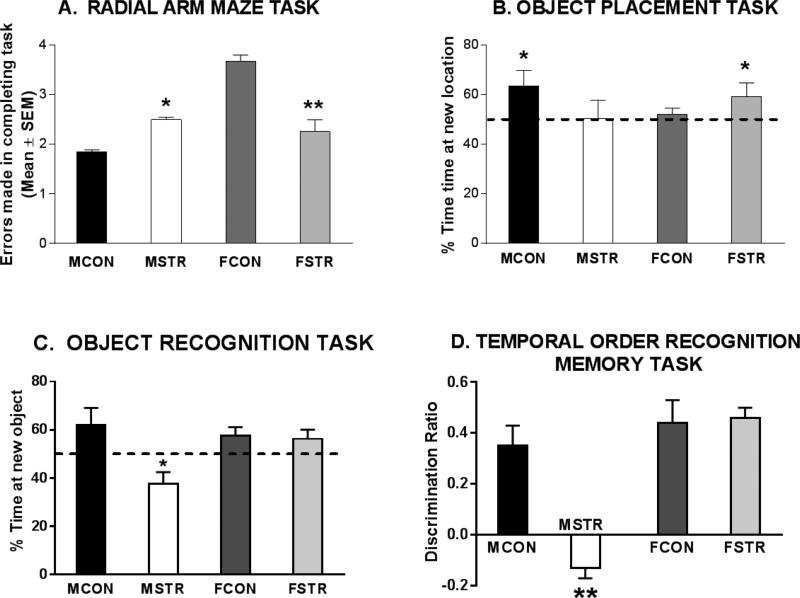
Figure 1. Sex differences in chronic stress effects on four different memory tasks.
A. Radial-arm Maze Task.
Subjects received 21 days of daily 6h restraint stress. Experiments were conducted separately in the sexes, and data for males (Luine et al, 1994) and females (Bowman et al, 2001) is combined in the figure. Estrus cycle stage was not determined in subjects. The number of errors made in completing the task significantly increased in stressed males and decreased in stressed females which suggests that stress impairs male and enhances female radial arm maze performance.
B. Object Placement Task.
Subjects received 21 days of daily 6h restraint stress. The percent time exploring objects in the new location is shown. Dotted line at 50% on the y-axis indicates chance performance of the task, i.e. subjects did not discriminate between the old vs the new location of the objects, which suggests poor remembrance of the old location. Control males and stressed females could significantly discriminate the old from new location but stressed males and control females could not which suggests that stress impairs male and enhances female spatial memory (redrawn from Beck and Luine, 2002).
C. Object Recognition Task.
Male and female rats received daily 6h restraint stress for 7 days. Entries are the percent time spent exploring the new object. Dotted line at 50% on the y-axis indicates chance performance of the task, i.e. subjects did not discriminate between the old vs the new objects which suggests poor remembrance of the old object. All groups except the stressed males spend significantly more time exploring at the new object suggesting that stress impairs male but does not affect female object memory. Data from Bowman et al (2009).
D. Temporal Order Recognition Memory (TORM task).
Subjects received 2h of daily restraint for 5-7 days. Entries are the discrimination ratio (time exploring the novel (less recent) object/time exploring all objects). Both control and stressed females spent more time exploring the novel object (high discrimination ratio) whereas only control, but not stressed, males spent more time exploring the novel object. This pattern suggests that stress impairs male, but not female, temporal order recognition memory (Redrawn from Wei et al, 2014).
For memory tasks which rely on prefrontal cortex input to learn/solve the task, a similar pattern emerges in male rodents, that is, stressors given from 1-3 weeks impair performance. For example, object recognition, a non-spatial task, is impaired following chronic stress to males. One – three weeks following daily restraint, stressed male rats are unable to significantly discriminate between known and new objects, an impairment of object recognition memory (See left two bars of Fig 1C) (Beck and Luine, 1999, 2002; Bowman et al, 2009; Gomez et al 2012). Temporal order recognition memory (TORM) is also impaired by 2 h of daily restraint for 5-7 days in male rats (Wei et al, 2014) (See left two bars of Fig 1D). TORM is similar to object recognition but uses two sampling periods in order to assess explicit memory processes which are dependent on the prefrontal cortex.
Thus, above cited and other studies show that chronic stress impairs both learning and memory in male rodents using a variety of tasks. A more extensive discussion of this subject can be found in Conrad, 2010 where results of studies are reviewed in relation to acquisition (learning), memory and other details including the nature of the tasks utilized. Conrad made similar conclusions: the vast majority of studies show that chronic stress impairs cognition in male rodents, but these effects can be moderated depending on type of tasks employed and the type and duration of stress.
2.2 Learning and memory is enhanced in females following chronic stress
Because estrogens are growth inducing in target tissues, exhibit neurotrophic, antioxidant and anti-apoptotic effects and also promote some aspects of cognitive function (Luine, 2015b), we hypothesized that female rats might be less sensitive than males to the effects of chronic stress. This idea was tested by giving adult females the same daily 6-hr restraint treatments and cognitive testing as males. Surprisingly, females were not just less sensitive to impairments, chronic restraint stress led to enhanced performance on some tasks. As shown in Fig. 1A for RAM testing (two bars on right side), females stressed for 21 days made fewer errors in completing the task than non-stressed females, and they also more rapidly reached learning criteria (Bowman et al, 2001). Similarly, 21 days of restraint stress enhanced female rodent performance in the MWM (Kitraki et al, 2004) and UCS did not affect female performance on the radial arm water maze (Ortiz et al, 2008). For spatial memory, as assessed by the object placement test (shown in the two right hand bars of Figure 1B), control females could not discriminate between the objects in the new vs the old location whereas females receiving restraint stress for 1-3 weeks did significantly discriminate which indicates improved memory following stress as compared to non-stressed controls (Beck and Luine, 2002; Bisagno et al, 2004). In the Y-maze, memory is either not affected or enhanced by 7-21 days of restraint stress in females (Conrad et al, 2003; Gomez and Luine, 2014). Also illustrated in Figures 1A and B is that RAM and object placement performance is better in non-stressed males than in non-stressed females. Females make approximately twice as many errors, four, as males to complete RAM (Fig. 1A), and control females are unable to significantly discriminate in object placement, spending an equal amount of time exploring at the old and new location of the objects (Fig. 1B). Superiority of male vs female rats in spatial memory tasks has been widely reported and is also present in humans (Luine, 2014a). Chronic stress appears to eliminate this sex difference by impairing male and enhancing female performance.
A somewhat different, but still sexually differentiated, pattern emerges when non-spatial tasks are tested following stress. In general, males are impaired on these tasks following chronic stress while females are not affected by the stress. For example, chronic stress does not alter female performance on the object recognition task, while it decreases male performance (Fig. 1C) (Beck and Luine, 1999; Bowman et al, 2009, Beck and Luine, 2002; Bisagno et al, 2004; Gomez and Luine, 2014). Like object recognition, TORM is not affected by stress in females, but TORM is decreased in stressed males (Fig. 1D) (Wei et al, 2014)
3. Sex differences and allostatic load
It is important to note that stress effects on cognition in both sexes depend on the specific task tested and the amount and duration of stress. For RAM, one week of restraint stress did not affect male performance while two weeks of stress enhanced performance (Luine et al, 1996), and, as discussed above, 3 weeks of restraint stress impaired RAM. For object placement, one week of restraint stress did not affect memory in males while 3 weeks impaired memory (Beck and Luine, 2002; Gomez et al, 2012). In object recognition, either one or three weeks of stress impaired male memory (Beck and Luine, 1999; 2002; Gomez et al, 2013). Overall, the time course of stress effects on male cognition is consistent with the conclusions of Selye (1976) and others who showed that short-term stress elicits adaptive physiological responses but that continued stress exposure results in maladaptive responses. More recently McEwen introduced the concept of allostasis and allostatic load to describe this stress relationship (1993). Thus, acute or short term stress in males enhances cognition as an effective coping mechanism while continued stress imparts too large an allostatic load on the organism and stress becomes debilitating to both peripheral and central nervous systems and results in learning and memory impairments (as well as other detrimental neural changes, see McEwen et al, 2016 for further information). It should be noted that these theories of stress effects are based on investigations in only one half of the population, males. Results in Fig. 1 show a different impact of chronic stress in females; learning and memory is either not affected by or is enhanced following chronic stress. We hypothesized, consistent with the concepts of Selye or McEwen, that the time course of change from adaptive to maladaptive effects or for the accumulation of adverse allostatic load might be greater in females as compared to males. Thus, longer periods of stress or greater intensities of stress may be necessary for impairments of memory in female rats or mice. However, recent studies have not supported this notion since longer periods of stress failed to impair cognition in female rats: no impairments in RAM, object recognition or object placement were found after 28 (Bowman et al, 2001) or 35 (Bowman and Kelly, 2012) days of stress in females. Thus, female rodents still show resilience to chronic stress, at least in terms of learning and memory, at longer periods of stress then males. However, it should be noted that few experiments have been conducted in female rodents and that most experiments in females utilized restraint stress. Another consideration is whether females may be less affected by specific stressors, like restraint, than males. Further discussion of this topic can be found in Bowman et al, 2003, but examination of several physiological endpoints of stress including corticosteroid release, weight loss, other behaviors like anxiety and the presence of neural changes show that females respond to stressors. Thus, chronic stress effects on female cognition, and other behaviors, need further investigation to confirm resilience effects and to understand how females fit into the allostatic load concept.
4. Mechanisms contributing to sexually differentiated responses to chronic stress
4.1. Influence of psychological and performance parameters on cognitive responses to stress
An important question for this review is whether the sex differences found in learning and memory following stress reflect changes in mnemonic processes or sex differences in other parameters that could influence performance on cognitive tasks. Parameters which might be sexually dimorphic and influence cognitive testing include (1) sensory-perceptive properties like olfaction, vision, audition, touch, nociception etc. (2) regulatory processes including thirst, hunger, and body weight/composition (3) motor considerations such as activity and skill, and, importantly, (4) affective parameters like arousal, anxiety, and motivation. Detailed discussion of these factors is beyond the scope of this review, but a short consideration follows.
When behavioral psychologists develop cognitive tasks, extensive control experiments determine contingencies that influence task performance (see Olton and Samuelson, 1976, for example). Not surprisingly, male rodents are the target subjects when developing tasks, and most tasks that lessen the effects of non-mnemonic variables and enhance the effects of mnemonic variables are biased toward males. Therefore, the possibility exists that stress affects mnemonic variables in males but non-mnemonic variables in females leading to the erroneous conclusion that stress enhances cognition in females. This conundrum is also considered on a neural basis by Shansky (2015). Somewhat abrogating this notion, similar patterns of stress effects in the sexes were found using five different tasks, and the tasks have different performance bases. The water maze is aversive (possibility of drowning) whereas the radial arm maze is rewarding (food) in order to complete the task. Recognition memory tasks and the Y-maze do not depend on reinforcement but instead utilize exploratory drive of subjects to complete the task. The demonstration of consistent sex differences in stress effects across these differently motivated tasks provides support that non-mnemonic factors in category (1) and (4) may not be primary in the sexually dimorphic responses.
In relation to regulatory processes of category (2), stress elicits decreases in weight gain across 21 days in both males and females, but losses are present earlier and larger in males than in females (Beck and Luine, 2002; Bowman et al, 2009; Gomez et al, 2013; 2014). Females have more body fat which could also influence results in the radial arm maze where subjects are food deprived. Thus, regulatory differences by stress in the sexes might contribute to enhanced performance of stressed females on the radial arm maze, but they do not appear to contribute to enhancements in recognition memory tasks. For (3), motor considerations, female rats are generally more active than males, but 21 days of daily restraint did not alter activity in either sex at adulthood or at an advanced age (File 1987, Beck and Luine, 2002; Bowman, 2006). Thus, changes in overall activity do not appear to have influenced the cognitive changes in these studies. In contrast, 7 days of daily restraint increased female, but decreased male, activity (Bowman et al, 2009), and the stressed males showed decreased object recognition memory (but not object placement memory) and the stress females showed increased object placement memory (but not object recognition memory). Thus, activity changes may have had some impact on cognitive testing following the 7 day stress regimen.
Of the affective parameters in category (4), little is known about chronic stress effects on arousal; however, it has been extensively studied following acute stress. In contrast, anxiety has been frequently assessed following chronic stress, and high anxiety levels can adversely affect learning and memory, especially in tests relying on exploration like recognition memory tasks. Moreover, sex differences in this behavior may also contribute to the sex differences in cognitive testing in non-stressed subjects. Adult males are generally more anxious and less active than females when assessed on the open field or in elevated plus maze (File et al, 1987, Beck and Luine, 2002; Bowman et al, 2009). Overall, chronic stress generally increases anxiety in both sexes as assessed by either the elevated plus maze or the open field (Luine et al, 2016). Thus, since stress increases anxiety in both sexes but impairs male memory (radial arm maze, object placement, object recognition) and enhances female memory (radial arm maze and object placement), anxiety-dependent changes may not underlie the cognitive changes. Anxiety may, however, influence sex differences in unstressed rodents. Yet, there were no sex differences in exploration times in the recognition memory tasks suggesting no differences in anxiety for objects. In support of this conclusion, Wright and Conrad (2005), using the Y-maze, showed that 21 days of chronic restraint in males impaired performance on a spatial version of the maze, but the subjects performed well in an intra-maze cued version which indicates that the cognitive deficit was not attributable to neophobia. In aged rats, stress caused a different effect on anxiety; males became more anxious and females became less anxious as indexed by open arm entries on the elevated plus maze (Bowman et al, 2006). Since both sexes showed improved object recognition following stress, changes in anxiety do not appear critical in the performance of this age group.
While changes in non-mnemonic parameters may contribute to sex differences in stress effects on spatial memory, the preponderance of current evidence does not support a major role for non-mnemonic factors in conferring sex differences in cognition nor in underlying sex differences in cognitive responses to stress. However, further research is necessary to substantiate this view.
4.2. Role of sex differences in strategies on cognitive responses to stress
Another factor which may contribute to sex differences in cognitive responses to stress is the use of different learning strategies in relation to sex, stress, or both. Both humans and rodents use multiple learning and memory systems in the brain which can be “switched on or off” (White and McDonald, 2002). One system involves the hippocampus which processes associative relations from a spatial-contextual representation and another involves the caudate nucleus which uses a stimulus-response procedural representation. Most simply, the hippocampal system forms memories of where things are in space (item A is in northeast area of the room) and the caudate system uses procedural relations (item A is located to the right of item B). Important for the current discussion is that the sexes may rely differently on these systems and that stress may impact use of these systems.
Sex differences in the proportion of individuals (humans and rodents) that exhibit a propensity to use a stimulus-response versus spatial learning strategy have been documented, and the proportion changes when the individuals are stressed. For instance, most male mice will exhibit a learned spatial strategy in a hole-board escape task, unless they are stressed prior to initial training, at which point a significant proportion will instead learn a stimulus-response strategy (Schwabe et al, 2010). In humans, women tend to use local visual information (stimulus – response) versus distal spatial information, whereas men use both strategies (Banta Lavenex, Lavenex, 2010); however with stress exposure a similar shift to stimulus – response strategy bias has been observed in a card-location task, as was reported in the animals (Schwabe et al, 2008). Based mainly on human studies, but also on some animal research, it has been hypothesized that stress may cause a uniform shift in neural processing which leads to altered cognitive function from a spatial/place to a response based strategy (Schwabe et al, 2008). In addition, when females were tested separately, both women (Hussain et al, 2016) and rats (Korol, 2004) exhibited cycle-dependent performance effects on tests that can be solved either though spatial or non-spatial strategies. These sex differences are not apparent prior to puberty as there is a general spatial bias (Kanit et al, 2016). As shown by Korol (2004), rather extreme biases in strategy use exist during high versus low periods of circulating ovarian hormones, or also by manipulating ovarian hormone levels. Similar shifts in strategy use are suggested by a recent study in women across the menstrual cycle (Hussain et al, 2016). Thus, it is possible that female resilience to stress is based on their use of both spatial and response strategies while males tend to use predominately spatial strategies. Since stress may cause a shift to response strategies, females show resilience but males do not. Thus, as we have discussed in the past (Beck & Luine, 2010), the enhancement or impairment in learning and memory following stressor exposure appears to depend upon what is being learned and whether it matches an individual's natural biases.
Shifts in processing style rely on activity of monoaminergic systems in the amygdala which in turn interacts with prefrontal cortex (PFC) and hippocampal areas (Packard et al, 1994). We have previously documented sex differences in stress effects on monoaminergic systems (See Luine et al, 2016 for review), and further discussion of these speculative concepts can be found in Beck and Luine (2010). Most notable, however, are documented changes in the physical connectivity in particular brain circuitry following stressor exposure, which occurs in the same brain regions implicated in driving the learning biases. For example, reduced hippocampal apical dendrites are seen in the male CA3 sub-region following chronic stressor exposure or repeated exogenous glucocorticoid exposure (Galea 1997) may work in conjunction with noradrenergic signaling in the amygdala to create the biological networks that force a shift in processing from a hippocampal spatial/place bias to a striatal stimulus-response bias (Packard 2009). These relationships are still speculative because of an inability to demonstrate dendritic arborization changes in vivo in humans, but the parallels are compelling. Thus, the contribution of strategy use by the sexes to differences in cognitive responses and stress resilience of females requires extensive investigation, but this theory is gaining momentum (Cahill, 2014).
4.3. Role of estrogens in sex differences in stress responses and female resilience
Because females have high circulating estradiol levels as compared to males, we tested the simple prediction that ovariectomized (OVX) females would not show stress resilience, i.e. be impaired on RAM following chronic stress, because of the loss in circulating estrogens. As shown in Fig. 2A, OVX + stressed rats’ performance was neither enhanced nor impaired as compared to non-stressed OVX females which partially supported the hypothesis that estradiol confers stress resilience (Bowman et al, 2002). However, McLaughlin et al (2005) showed that chronically stressed OVX rats still showed some improvements on the Y-maze as compared to non-stressed subjects. Consistent with our hypothesis, when estradiol was given to OVX-stressed females, RAM performance was enhanced (Fig. 2A) (Bowman et al, 2002). We then hypothesized that stress resilience might be due to both activating effects of estradiol at adulthood and also enduring, organizing effects of estradiol which occur during development in females and are expressed at adulthood even without the presence of estradiol. However, it is now established that estradiol is synthesized locally in discrete regions of the brain by the enzyme aromatase from androgen precursors such as testosterone and/or directly from cholesterol. Thus, estradiol levels in the hippocampus, hypothalamus and prefrontal cortex are higher than in the circulation and may confer resilience to stress. Kato et al (2013) reported hippocampal 17β-estradiol levels of 4 nM in female rats at proestrus while circulating concentrations were only 0.1 nM. Most important for this discussion, estradiol remains in the hippocampus following ovariectomy due to this local synthesis (Kato et al, 2013) and could therefore mediate stress resilience in the hippocampus of females. Surprisingly, investigations in hippocampal slices showed that both sexes synthesize estradiol, but it appears that locally derived hormone is not effective in males since estradiol maintains LTP and synapses in females but not males, and inhibition of estradiol synthesis results in LTP impairment and synapse loss in females but not males (Fester and Rune, 2015) Thus, in situ production of estradiol and its sexually dimorphic effects within the hippocampus indicates that neural estradiol can contribute to cognitive stress resilience in females such that estradiol is available to hippocampal and other sites even following ovariectomy , but the basis for the apparent lack of effects in males remains to be investigated.
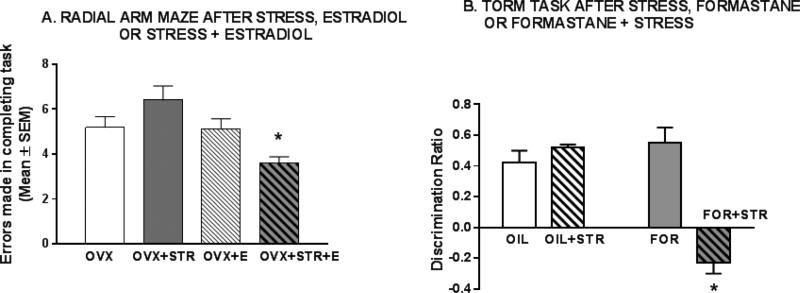
Figure 2. Effects of decreasing estradiol levels on stress outcomes in females.
A. Radial arm maze task and effects of ovariectomy.
Rats were ovariectomized and served as control (OVX), were restraint stressed 6 h daily for 21 days (OVX + STR), received estradiol in Silastic capsules (OVX + E) or were stressed daily and received estradiol (OVX + STR + E). ANOVA showed main effects of estradiol treatment (p < 0.003) and an estradiol-stress interaction (p < 0.002) indicating that estradiol treated groups showed better performance and the estradiol-treated stressed females outperformed all other groups (p < 0.05). The pattern of changes suggests that peripheral estradiol, provided from the ovaries, provides resilience to stress. (Redrawn from Bowman et al, 2002).
B. Temporal order recognition task and effects of aromatase inhibition.
Female mice received S.C. OIL, OIL and 7 days of 6 h daily restraint (OIL + STR), Formastane (FOR, a drug which decreases estrogen production in the brain and ovaries by inhibiting the enzyme aromatase) and Formestane 1 hr. before a daily stress (FOR + STR). Data are discrimination ratios (see 1D). All groups significantly discriminated except FOR + STR suggesting that peripheral and neural derived estradiol provide resilience to stress. (Redrawn from Wei et al, 2014).
Circulating estradiol levels are low in females at adolescence and in old age; thus, lack of resilience to stress during these ages might provide indirect evidence that estradiol contributes to cognitive resilience. Generally, these time periods have not been well examined, but we did find that 21-month old females no longer showed enhanced OP following chronic stress like young, adult females (Bowman et al, 2006). Adolescent experiments have either not examined cognition or not measured both sexes, but, interestingly, Bourke and Neigh (2011) found that stressed, adolescent females, but not males, showed greater depressive behavior during adolescence and at adulthood. Why males were not affected is not clear, but experiments during this time period are complicated because the hippocampus is not fully developed at adolescence.
Specific evidence that estradiol provides cognitive resilience to stress in females was provided by Zhen and colleagues who recently explored sex differences in response to stress in the TORM task (Wei et al, 2014). As shown in Fig. 1D, chronic restraint stress impairs male, but not female mice, in the TORM task. However, when estrogen receptors were inhibited or knocked out in the prefrontal cortex of females, stressed females were impaired in TORM. Likewise, impairments of performance in stressed males were prevented by estradiol administration suggesting that peripheral (gonadally derived?) estradiol may also confer resilience to stress, because as discussed above, neural estradiol does not appear to be active in the hippocampus of males. To further test involvement of neural estrogen, females were injected S.C. with the aromatase inhibitor, Formestane, which blocked both peripheral and neural synthesis of estradiol (Wei et al, 2014). Following this treatment, stress impaired TORM in females (Fig. 2B). When taken together with experiments on RAM and Y mazes in females, where only peripheral estrogen was removed by OVX (described above), these results provide some evidence that both peripherally and neurally derived estradiol provide resilience in females against the detrimental effects of chronic stress on memory.
5. Contributions of sex differences in the stress response to mental health
The resilience to stress of female rodents does not appear consistent with the general behavioral phenotype of human females who show more stress related disorders such as depression, anxiety and posttraumatic stress disorder than males (Bangasser and Valentino, 2014). Several possibilities for this discrepancy between species have been advanced including that rodents may not successfully model humans, but a recent study showing greater depressive behaviors in female, but not male, mice after one week of CVS suggests that mice may be more appropriate models than rats (cognition was not investigated) (Hodes et al, 2016). Another factor discussed above is that current behavioral tests in rodents were developed in males, and thus, results in females may not accurately tap into feminine attributes. On the other hand, it can be envisioned that heightened cognition in females following stress might facilitate remembrance of stressful events better than in cognitively impaired males and combined with enhanced anxiety may, at a longer time interval post stress, culminate in more depression and PTSD in females. Thus, future experiments should assess stress-dependent effects at longer intervals following cessation of stress.
Another consideration for research is that chronic stress is often coincident with other behaviors such as increased alcohol and drug consumption which may serve as a “self-medication” to mitigate the “stressed or burned out” feelings. Thus, our laboratories became interested in exploring possible relationships between stress, sex and alcohol in adult rats. First, we compared alcohol consumption in male rats that were unstressed or received 6 h/day of restraint stress. Using a two-bottle choice (one bottle with water and the other with 8% alcohol), limited access paradigm for one hour daily, we found that stressed rats showed greater alcohol consumption as compared to the alcohol only group, an effect seen in several but not all, previous studies (Gomez, et al, 2012). We also tested object placement and found, as in previous studies, that stressed only rats and alcohol only rats were impaired, but the alcohol + stressed rats performed as well as the control group (no stress, no alcohol). This result shows that the combination of alcohol and stress reverses the individual, deleterious effects of stress or alcohol. Whether alcohol blocks or mitigates the stress effect, or vice versa, is not known, but these results show that stressed rats increase alcohol consumption and suggest that the effects may be like in men who self-medicate with alcohol to relieve stress-related effects (Sinha, 2001).
We further investigated the interactive effects of stress and alcohol on cognition in male as well as female rats. As there was individual variability in the amount of consumption in the two-bottle paradigm, we utilized gavage of alcohol in order to deliver a constant dose. For seven days, subjects were controls, restrained for 6 hours, gavaged with alcohol (2.0 g/kg), or restrained for 6 hrs and then received alcohol gavage (Gomez et al, 2013; Gomez and Luine 2014). Consistent with previous stress (Beck and Luine, 1999; Bowman 2009) or alcohol studies (Ryabinin et al, 2002), stress alone or alcohol alone impaired object recognition (Figure 3A). Male rats in these groups spent equal time exploring at the old and new objects, but, surprisingly, the stress + alcohol group, like the control group, spent significantly more time exploring at the new, compared to the old (known) object. A similar pattern was seen for the Y-maze where the alcohol alone and stress alone groups showed no differences between exploration of the arms while the control and stress + alcohol groups explored the novel arm more than the other and start arm. Thus, in male rats for both a spatial and non-spatial memory task, there was an interactive effect between stress and alcohol that spared memory impairments caused by each treatment alone. A different pattern of results was found in female rats. Control and stressed females spent more time exploring the novel object, consistent with previous studies (Beck and Luine, 1999; 2002; Bisagno et al, 2003; Bowman, et al, 2009) while the alcohol and stress + alcohol groups spent the same amount of time exploring old and new objects (Figure 3B). Thus, alcohol appears to impair object memory, and, unlike in males, the combination of stress + alcohol does not spare alcohol-dependent memory impairments in females. For Y-maze testing, only the control females showed greater exploration of the novel arm compared to the other and start arms (Gomez et al, 2014). Thus, alcohol, stress or the combination impaired female spatial memory on the Y-maze, a different pattern than in males where the combination treatment spared the impairing effects of alcohol or stress. Taken together, these experiments examining the interactive effects of stress and alcohol on cognition indicate that robust sex differences are present not only in responses to stress alone but that the combination of stress with alcohol results in a further delineation of sex-dependent responses. For the cognitive realm, the combination of alcohol with stress appears to alleviate the stress induced impairments in memory, both spatial and non-spatial, in males. However in females, stress normally has no effect on memory (object recognition) or enhances some spatial memory tasks, but combining alcohol with stress, results in impairments in both types of memory.
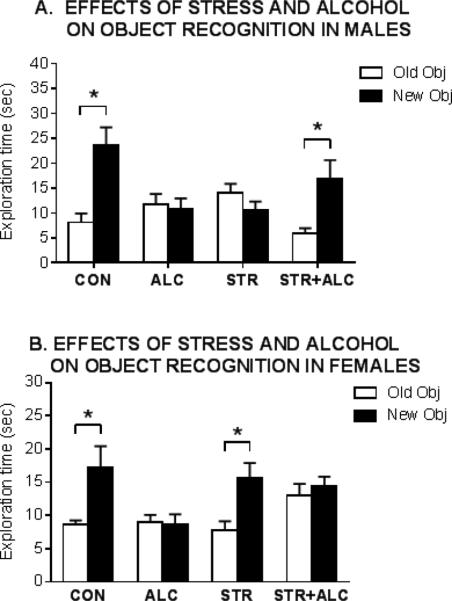
Figure 3. Sex differences in the effects of stress, alcohol and combined stress + alcohol on object recognition memory.
Adult male and female rats received 7 days of 6 h daily restraint. Exploration time for old and new objects during the retention trial is shown. Greater exploration of the new vs the old object indicates that subjects remember the previously viewed (old) object.
A. Effects in male rats
The CON and STR + ALC groups explored the new objects more than the old objects, p < 0.05, but the ALC and the STR groups explored the old and new objects for the same amount of time. This result suggests that ALC or STR impairs object memory and that the combination of STR + ALC reverses the impairing effects on memory of either treatment alone. (Redrawn from Gomez et al, 2013).
B. Effects in Female rats
The CON and STR groups explored the new objects more than the old objects, p < 0.05, but the ALC and the STR + ALC groups explored the old and new objects for the same amount of time. This result suggests that stress does not alter object memory but that ALC impairs object memory, and further, stress does not reverse the impairing effect of alcohol. Estrus cycle stage was not determined. (Redrawn from Gomez et al, 2014).
These results may help to explain some sex differences in mood disorders and drug use/abuse. The observation that stress dependent cognitive deficits were reversed in males, but not females, when alcohol was given following the stress is consistent with the observation that males abuse drugs and alcohol more than females. This relationship suggests that drug therapies for stress related disorders should be examined within the context that different effects may result depending on the sex of the subject. Amphetamine and chronic stress also interact to alter behavioral and neural responses in females (Bisagno et al, 2004) but possible interactive effects in males have not been directly compared to females. Overall, studies in rodents that utilize stress combined with drugs or alcohol might provide more consistency with human conditions.
5. Conclusions
While sex differences in cognitive responses to stress have been demonstrated in rats, the relationship of these effects to sex differences in the incidence of stress related diseases in humans remains largely unknown. However, more information about sex differences in neural function and behavior may be forth coming since the National Institutes of Health (NIH) has recently unveiled policies to ensure that preclinical research includes subjects of both sexes (Clayton and Collins, 2014). As reviewed here, most research does not include both sexes, and we have only scratched the surface on how stress is processed in female versus male brains. We posit that only by using both sexes will it be possible to forge meaningful links between stress and mental functioning.
Highlights.
Chronic stress impairs cognition in male rodents
Chronic stress does not affect or enhances female cognition depending on contingencies of the task
Estrogens provide cognitive resilience to stress in females
Cognitive sex differences may contribute to differential incidences of stress-related diseases
Acknowledgments
Experimental work discussed in this review was supported by NIH grants GM60654 (VL), GM60665 (VL) and MD007599 (HC), the City University of New York and PSC-CUNY. The authors thank the many undergraduate and graduate students who participated in the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–19. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta Lavenex P, Lavenex P. Spatial relational learning and memory abilities do not differ between men and women in a real-world, open-field environment. Behav Brain Res. 2010;207:125–37. doi: 10.1016/j.bbr.2009.09.046. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999;830:56–71. doi: 10.1016/s0006-8993(99)01380-3. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol. Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Beck K, Luine VN. Evidence for sex specific shifting of neural processes underlying learning and memory following stress. Physiology & Behavior. 2010;99:204–211. doi: 10.1016/j.physbeh.2009.04.011. PMC in Progress. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Chronic d-amphetamine induces sexually dimorphic effects on locomotion, recognition memory and brain monoamines. Pharmac. Biochem. & Behav. 2003;74:859–867. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Grillo CA, Piroli GG, Giraldo P, McEwen B, Luine VN. Chronic stress alters amphetamine effects on behavior and synaptophysin levels in female rats. Pharmac Biochem Behav. 2004;78:541–50. doi: 10.1016/j.pbb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Bowman RE. Stress-induced changes I spatial memory are sexually differentiated and vary across the lifespan. J. Neuroendo. 2005;17:526–535. doi: 10.1111/j.1365-2826.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm. Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman R, Maclusky NJ, Zrull MJ, Diaz SE, Luine VN. Sex Differences in Aged Rats: Behavioral and Physiological Responses to Chronic Stress. Brain Research. 2006;1126:156–166. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex dependent changes in anxiety, memory, and monoamines following one week of stress. Physiology & Behavior. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Kelly R. Chronically stressed female rats show increased anxiety but no behavioral alterations in object recognition or placement memory: a preliminary examination. Stress. 2012;15:524–32. doi: 10.3109/10253890.2011.645926. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Equal = the same: sex differences in the human brain. Cerebrum 5. 2014 eCollection. [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:742–55. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KD, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Fester L, Rune GM. Sexual neurosteroids and synpatic plasticity in the hippocampus. Brain Res. 2015;1621:162–9. doi: 10.1016/j.brainres.2014.10.033. [DOI] [PubMed] [Google Scholar]
- File SE, Curle PF, Baldwin HA, Neal MJ. Anxiety in the rat is associated with decreased release of 5-HT and glycine from the hippocampus. Neurosci. Lett. 1987;83:318–322. doi: 10.1016/0304-3940(87)90107-8. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Lewis M, Luine V. Alcohol Access Alleviates Stress Induced Spatial Memory Impairments in Male Rats. Alcohol. 2012;46:499–504. doi: 10.1016/j.alcohol.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Lewis MJ, Sebastian V, Serrano P, Luine V. Alcohol Administration Blocks Stress-Induced Impairments in Memory and Anxiety and Alters Hippocampal Neurotransmitter Receptor Expression in Male Rats. Hormones & Behavior. 2013;63:659–661. doi: 10.1016/j.yhbeh.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Luine V. Female Rats Exposed to Stress and Alcohol Show Impaired Memory and Increased Depressive-like Behaviors. Physiology and Behavior. 2014;123:47–54. doi: 10.1016/j.physbeh.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain D, Hanafi S, Konishi K, Brake WG, Bohbot VD. Modulation of spatial and response strategies by phase of the menstrual cycle in women tested in a virtual navigation task. Psychoneuroendocrinology. 2016;70:108–117. doi: 10.1016/j.psyneuen.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Histoffel DJ, et al. Russo SJ. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to sub-chronic variable stress. J. Neurosci. 2015;35:16362–76. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz OA, Balkan B, Demirgören S, Furedy JJ, Pögün S. Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res Bull. 2016;52:243–8. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004;125:47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiology of Learning & Memory. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Luine V. Recognition memory tasks in neuroendocrine research. Behav Brain Res. 2015a;285:158–164. doi: 10.1016/j.bbr.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol: mediator of memories, spine density and cognitive resilience to stress in female rodents. J. Steroid Biochemistry and Molecular Biolog. 2015b doi: 10.1016/j.jsbmb.2015.07.022. S0960-0760(15)30034-0. 2015.07.022. [Epub ahead of (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Gomez JL. In: Sex Differences in Rodent Cognitive Processing and Responses to Chronic Stress in Sex Differences in The Central Nervous System. Shansky Rebecca., editor. Elsevier; 2015. pp. 365–404. [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen B. Restraint stress reversibly enhances spatial memory performance. Physiology and Behavior. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Luine V, Gomez J, Beck K, Bowman R. Sex differences in chronic stress: Role of estradiol in cognitive resilience. In: Fink George., editor. Neuroendocrinology and Endocrinology; Volume 2 of the Handbook of Stress Series. Elsevier; 2016. [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–101. [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Paris JJ, Mitzelfelt MS, McDonough S, Frye CA, Matuszewich L. Sex-dependent effects of chronic unpredictable stress in the water maze. Physiol Behav. 2011;102:266–75. doi: 10.1016/j.physbeh.2010.10.022. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–54. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J. Experimental Psych Animal Behav. Proc. 1976;2:97–116. [Google Scholar]
- Ortiz JB, Taylor SB, Hoffman AN, Campbell AN, Lucas LR, Conrad CD. Sex-specific impairment and recovery of spatial learning following the end of chronic unpredictable restraint stress: potential relevance of limbic GAD. Behav Brain Res. 2015;282:176–84. doi: 10.1016/j.bbr.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiology of Learning and Memory. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG. Anxiety, cognition, and habit: a multiple memory systems perspective. Brain Res. 2009;1293:121–8. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh L. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. PNAS USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Miller MN, Durrant S. Effects of acute alcohol administration on object recognition learning in C57BL/6J mice Pharmacol. Biochem. Behav. 2002;71:307–312. doi: 10.1016/s0091-3057(01)00661-x. [DOI] [PubMed] [Google Scholar]
- Schwabe R, Dalm S, Schachinger H, Oitzl MS. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neuobiol. Learn. and Memory. 2008;90:495–503. doi: 10.1016/j.nlm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Schächinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as a switch between memory systems. J Cogn Neurosci. 2010;7:1362–72. doi: 10.1162/jocn.2009.21278. [DOI] [PubMed] [Google Scholar]
- Selye H. The Stress of life. McGraw Hill; New York: 1976. [Google Scholar]
- Shansky RM. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress. 2015;1:60–65. doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic Stress, drug use and vulnerability to addiction. Ann. NY Acad. Sc. 2008;1141:105–130. doi: 10.1196/annals.1441.030. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. Psychiatry. 2014:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RB. Theoretical Review: Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory. 2002;77:125–84. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–4. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]