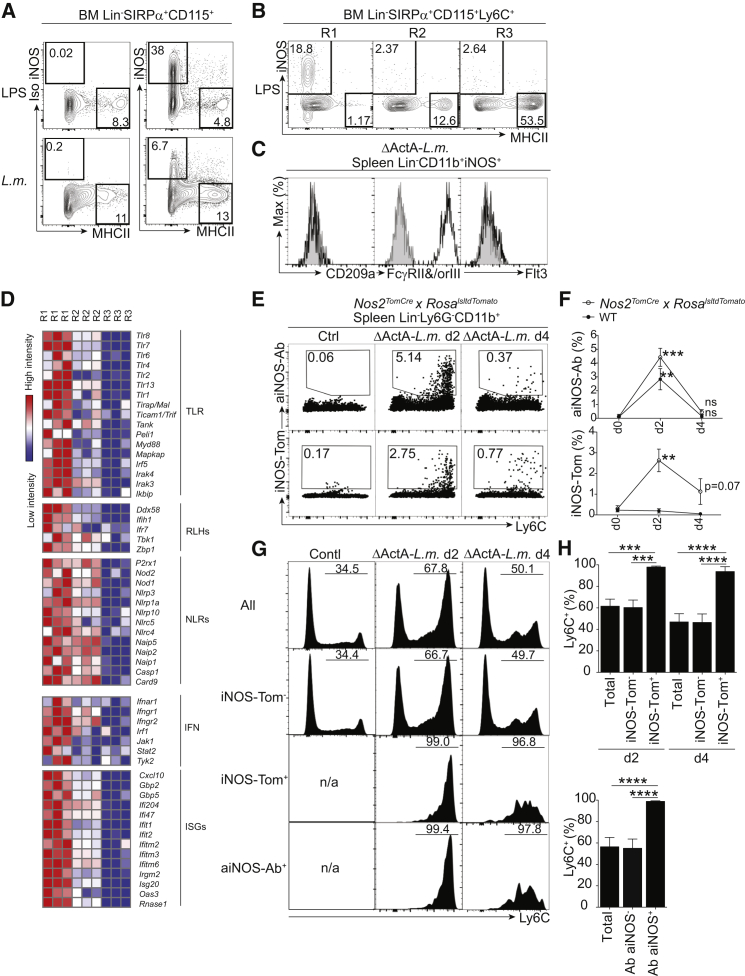
Figure 4.
Pu.1loFlt3−MHCII− R1 Monocytes Differentiate into iNOS+ Phagocytes upon Microbial Stimulation
(A) iNOS production by CD115+ cells in vitro. Shown is surface MHCII and intracellular iNOS or isotype control (Iso iNOS) staining after overnight culture of BM Lin−CD115+ cells in the presence of LPS or L.m. (MOI = 0.1).
(B) In vitro microbial stimulation of R1–R3. Shown is the analysis of surface MHCII and intracellular iNOS on sorted R1–R3 cells cultured overnight in the presence of LPS (1 μg/ml).
(C) Cell-surface phenotype of Lin−CD11b+iNOS+ cells during L.m. infection (day 2). Shown is flow cytometry analysis of CD209a, FcγRII and/or FcγRIII (CD16/32), and Flt3 (black lines) against respective isotype controls (gray shading).
(D) Pathway analysis of differentially expressed genes in flow-cytometry-sorted steady-state BM R1–R3. Abbreviations are as follows: TLR, toll-like receptor; RLH, RIG-like helicase; NLR, NOD-like receptor; IFN, interferon; and ISG, interferon-stimulated gene.
(E–H) Fate mapping of L.m.-induced iNOS-expressing splenocytes. (E) Nos2TomatoCre x RosalsltdTomato mice infected with the ΔActA mutant of L.m. were analyzed for intracellular anti-iNOS staining (top) and tomato labeling (bottom) in Lin−CD11b+ splenocytes in control or L.m.-infected mice (days 2 and 4). (F) Mean ± SEM of the percentage of iNOS-Ab+ (top) and Tomato+ (bottom) cells in Bl/6 (WT) or Nos2TomatoCre x RosalsltdTomato mice in untreated (d0) or L.m.-infected mice on days 2 and 4 (n = 3). (G) Histograms show Ly6C expression in cells either stained or unstained with anti-iNOS antibody or iNOS-Tomato. (H) Percentages of Ly6C+ cells within each indicated population (n = 4 mice per group; ∗∗p < 0.005,∗∗∗p < 0.0005, ∗∗∗∗p < 0.00005; Student’s t test).
Please also refer to Figure S4.