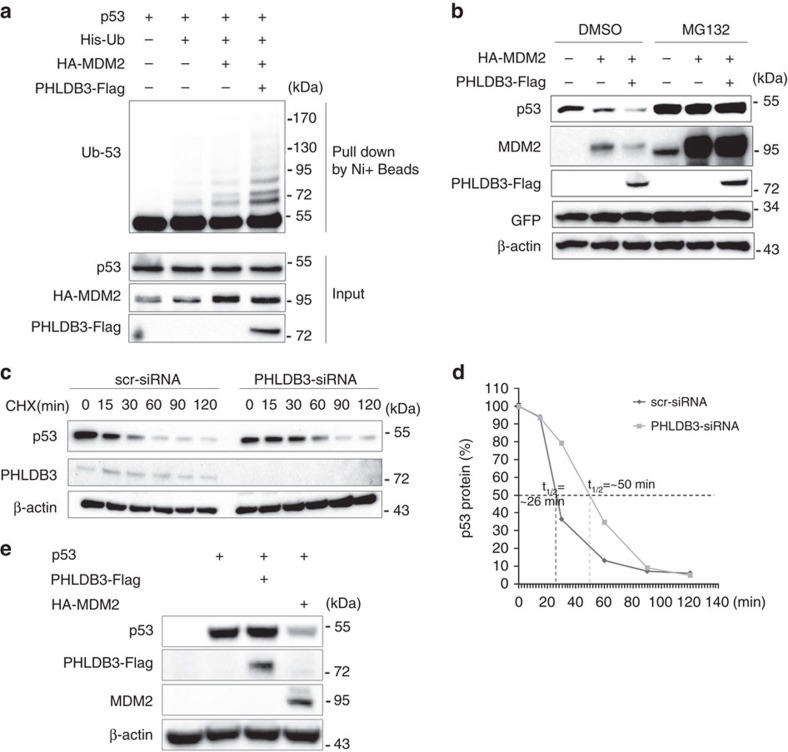
Figure 5. PHLDB3 promotes p53 degradation by enhancing MDM2-mediated ubiquitination.
(a) PHLDB3 promotes MDM2-induced p53 ubiquitination. HCT116p53−/− cells were transfected with combinations of plasmids encoding p53, HA-MDM2, PHLDB3-Flag or His-Ub, and treated with MG132 6 h before harvested for in vivo ubiquitination assay. Bound and input proteins were detected by immunoblotting using antibodies as indicated. (b) PHLDB3 enhances MDM2-mediated p53 proteasomal degradation. HCT116p53+/+ cells were transfected with combinations of plasmids encoding EGFP, HA-MDM2 or PHLDB3-Flag followed by immunoblotting using antibodies as indicated. MG132 was supplemented to the medium for 6 h. (c,d) p53-half-life is increased upon PHLDB3 knockdown. HCT116p53+/+ cells transfected with PHLDB3 or scramble siRNA for 72 h were treated with 100 μg ml−1 of CHX and harvested at different time points as indicated. The p53 protein level was detected by immunoblotting (c), quantified by densitometry and plotted against time to determine p53-half-lives (d). (e) Ectopically expressed PHLDB3 does not alter p53 protein level in the absence of MDM2. MEFp53−/−; Mdm2−/− cells were transfected with combinations of plasmids encoding PHLDB3-Flag, HA-MDM2 or p53 followed by immunoblotting using antibodies as indicated.