Abstract
Feeder cells are generally required for establishment and maintenance of embryonic stem (ES)/induced pluripotent stem (iPS) cells. Increased demands for generation of those cells carrying various types of vectors (i.e., KO vectors and transgenes) also require feeder cells that confer resistance to any types of preexisting selective drugs. Unfortunately, the use of the feeders that are resistant to various drugs appears to be limited to a few laboratories. Here we generated a set of gene-engineered STO feeder cells that confer resistance to several commercially available drugs. The STO cells, which have long been used as a feeder for mouse ES and embryonal carcinoma (EC) cells, were transfected with pcBIH [carrying bleomycin resistance gene (ble) and hygromycin B phosphotransferase gene (Hyg)], pcBIP [carrying ble and puromycin resistance gene (puro)], or pcBSN [carrying ble and neomycin resistance gene (neo)]. The resulting stably transfectants (termed SHB for pcBIH, SPB for pcBIP, and SNB for pcBSN) exhibited bleomycin/hygromycin, bleomycin/puromycin, or bleomycin/neomycin, as expected. The morphology of these cells passaged over 18 generations was indistinguishable from that of parental STO cells. Of isolated clones, the SHB3, SPB3, and SNB2 clones successfully supported the growth of mouse ES cells in an undifferentiated state, when coculture was performed. PCR analysis revealed the presence of the selective markers in these clones, as expected. These SHB3, SPB3, and SNB2 cells will thus be useful for the acquisition and maintenance of genetically manipulated ES/iPS cells.
Keywords: Feeder, STO cell, Selective drug, Plasmid, ES cell, iPS cell
Introduction
Embryonic stem (ES) cells have the ability to differentiate into virtually all cell types; therefore, they have enormous potential in tissue engineering, cell therapy disease models, and drug delivery studies (9,12,23). However, the therapeutic use of ES cells was limited by technical, ethical, or immunological considerations. To overcome these limitations, the use of induced pluripotent stem (iPS) cells has emerged based on the overexpression of selected four factors relevant to the pluripotent phenotype by somatic cell reprogramming (20,21). One benefit of these iPS cells is that it can eliminate immunological concerns associated with allogeneic or xenogeneic donor cells in clinical applications. Another benefit is that they largely remove the ethical concerns that limit the use of human ES cells. iPS cell research is now in its infancy, and so there are still many questions and problems that need to be clarified.
Feeder cells are strictly required for the derivation and maintenance of ES/iPS cells (10,20). However, they can neither accelerate reprogramming nor increase the generation frequency of iPS cell colonies (3). Since Nichols et al. (8) reported usefulness of primary cultured mouse embryonic fibroblasts (MEFs) as feeder cells for maintaining human ES cells, MEFs have been employed by many researchers (23,26). However, the use of MEFs has some drawbacks. First, it is often difficult to obtain MEFs transfected with exogenous DNA (i.e., drug resistance gene expression vector), because their proliferation ability is generally limited compared with that of other immortalized cells. Second, MEFs have to be isolated from midgestational fetuses, which are sometimes laborious, and the quality of MEF itself may often differ depending on the cell isolation skill of individuals. The Sandos inbred mice (SIM) 6-thioguanine-resistant, ouabain-resistant (STO) cell line, a line established from mouse SIM embryonic fibroblasts (24), has long been employed as feeder cells for the establishment and maintenance of ES cells (2,22). It lacks hypoxanthine guanine phosphoribosyltransferase activity and therefore is resistant to 6-thioguanine and ouabain, but sensitive to the hypoxanthine, aminoprotein, and thymidine selection. STO cells can continue to proliferate in vitro due to their immortalized nature. They are also known to cease to proliferate after treatment with mitotic inhibitor such a mitomycin C (MMC) (6). These properties appear to be superior to MEFs upon use as a feeder for generation of gene-engineered ES/iPS cells.
Increased demands for generation of those cells carrying various types of vectors [i.e., knockout (KO) vectors and transgenes] also require feeder cells that confer resistance to several types of selective drugs. Unfortunately, the use of the feeders that are resistant to several preexisting drugs appears to be limited. In this study, we generated gene-engineered STO feeder cell lines that confer resistance to any types of selective drugs.
Materials and Methods
Cell Culture
The STO cells (#1503, ATCC, VA) were cultured in Dulbecco's modified Eagle's medium (#11995-081, GIBCO®, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; #SFMB30-2239, Equitech Bio, TX), 50 U/ml penicillin, and 50 mg/ml streptomycin (#15140-122, Invitrogen, CA) in a humidified atmosphere of 5% CO2 in air at 37°C. Approximately 2 × 105 cells were seeded in 60-mm gelatin-coated dishes (Iwaki Glass Co. Ltd., Tokyo, Japan) the day before transfection.
Plasmid Construction
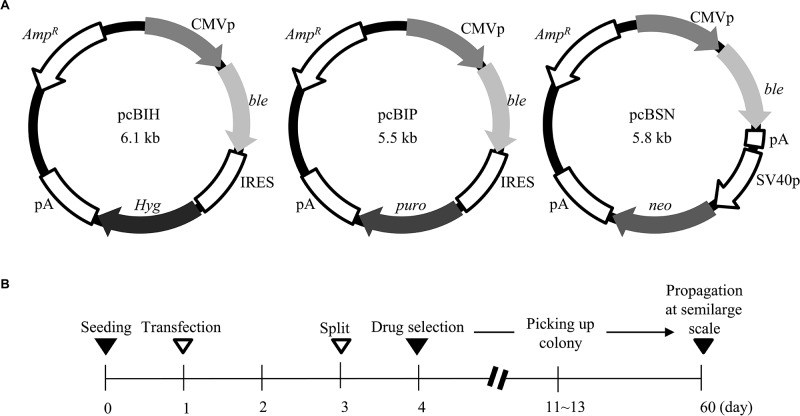
For the generation of gene-engineered STO cells that confer resistance to several types of selective drugs, three plasmids, termed pcBIH, pcBIP, and pcBSN (Fig. 1A) were constructed. The pcBIH plasmid was constructed by inserting ble derived from pIRES-bleo (#631622, Clontech Labs., CA) into the site between the cytomegalovirus (CMV) promoter and the internal ribosome entry site (IRES) sequence + Hyg. Similarly, the pcBIP plasmid was constructed by inserting ble into the site between the CMV promoter and the IRES + puro. The pcBSN plasmid has ble and neo, whose expression is driven by the CMV and SV40 early promoter, respectively. The IRES is a specific sequence that confers to generate two or more independent proteins from a single mRNA (11). Thus, cells transfected with pcBIH can survive in the presence of bleomycin and hygromycin B. Similarly, cells transfected with pcBIP will be resistant to bleomycin and puromycin. Cells transfected with pcBSN will be resistant to bleomycin and neomycin, because two types of drug-resistant proteins are produced from the two different expression units, as depicted in Figure 1A.
Figure 1.
(A) Structure of plasmids, pcBIH, pcBIP, and pcBSN. ble, bleomycin resistance gene; pA, poly(A) sites; Amp R, ampicillin resistance gene; CMVp, cytomegalovirus promoter; IRES, internal ribosomal entry site; puro, puromycin resistance gene; Hyg, hygromycin B resistance gene; neo, neomycin resistance gene; SV40p, SV40 early promoter. (B) Schedule for isolation of drug-resistant STO cells. Selection was performed 3 days after transfection in media containing bleomycin/hygromycin B (for cells transfected with pcBIH), bleomycin/puromycin (for cells transfected with pcBIP), or bleomycin/neomycin (for cells transfected with pcBSN).
Transfection
The STO cells were transfected with each of the plasmids (pcBIH, pcBIP, and pcBSN) mentioned above to obtain stable transfectants (termed SHB, SPB, and SNB, respectively). Transfection was performed by liposomal transfection methods described by Suzuki et al. (19) using Lipofectamin Plus (#11514-001, Invitrogen). Two days after transfection, cells were split into a 60-mm dish. On the following day, selection of the transfected cells was performed by incubating them in the presence of selective drugs, according to the schedule described in Figure 1B. The concentrations of drugs used are 40 μg/ml of hygromycin B (#10687-010, Invitrogen), 300 μg/ml of bleomycin (Zeocin; Invitrogen, R250-01), 25 μg/ml of puromycin (#A11138-02, Invitrogen), and 200 μg/ml of G418 (Geneticin; #10131-035, Invitrogen). In obtaining SHB, the transfected cells were selected with bleomycin and hygromycin B for 7–9 days. Similarly, SPB and SNB cells were obtained by selection of the transfected cells with bleomycin/puromycin or puromycin/neomycin, respectively. The resulting drug-resistant colonies (9–10) were picked up by a steel-ring method and propagated by passaging cells every 3 days for 2 months in the presence of selective drugs.
Mouse ES Cell Culture
Prior to seeding of the ES cells, the STO feeder layer was treated with 10 mg/ml MMC (Sigma-Aldrich, M4287, MO) for 2 h at 37°C and then washed three times with phosphate- buffered saline, pH 7.4. Mouse ES cells (#1002, ATCC, VA) were generally maintained in complete ES cell medium supplemented with 15% FBS (#ES-101-B, Millipore, MA) on the MMC-treated feeder layer. Cell passage was performed every 3 days, and the medium was changed daily.
Evaluation of the Ability of Stably Transfected STO Clones to Support ES Cell Growth
The gene-engineered STO-based feeder cells (Fig. 2) were first treated with MMC, as mentioned above. These cells (approximately 2 × 104 cells) were seeded onto a 35-mm gelatin-coated dish (#4000-020, AGC Techno Glass Co. Ltd.). Mouse ES cells (approximately 2 × 104 cells) were then seeded onto each transfected clone and cultured for 4 days. The culture was then split into five aliquots, of which one aliquot was seeded onto the feeders (approximately 2 × 104 cells) in a 35-mm gelatin-coated dish. This was done once again. Three to four days after seeding, ES cell-derived colonies, also termed as “moles” comprising cell aggregates characteristic to ES/iPS cells (as shown in Fig. 3A), are frequently observed, indicating that they are maintained in an undifferentiated state. On the other hand, clusters of differentiated cells (as shown in Fig. 3B), probably derived from ES cells, are also seen, although their frequency appears to be generally low. The dish containing more than 50% of moles per moles + clusters comprising differentiated cells was defined as the dish containing a feeder clone with the ability to maintain ES cells in an undifferentiated condition. The dish containing less than 50% of moles per moles + clusters comprising differentiated cells was defined as the dish containing a feeder clone that failed to maintain ES cells in an undifferentiated condition.
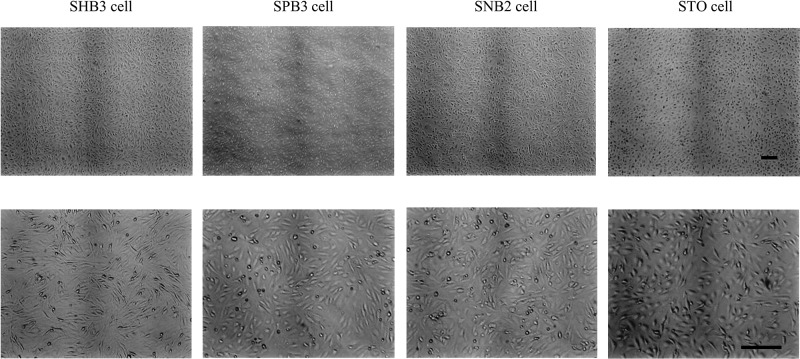
Figure 2.
Morphology of isolated drug-resistant STO clones, SHB3, SPB3, and SNB2. These cells are passaged over 18 generations. Note that all these cells exhibit similar morphology to their parental STO cells. Scale bar: 250 μm.
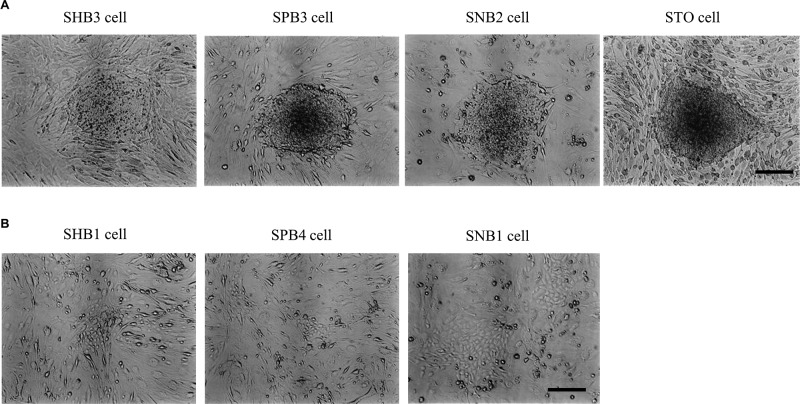
Figure 3.
(A) Embryonic stem (ES) cell aggregates grown on each of the stable STO transfectants (SHB3, SPB3, and SNB2). Note the presence of typical undifferentiated morphology of ES cells on the feeder cells. (B) Differentiated cells derived from ES cells upon seeding onto another stable STO transfectants. Scale bar: 250 μm.
Sensitivity Assay for Stably Transfected STO Clones
Some isolated drug-resistant STO cell clones were incubated in higher concentrations of drugs to see at how much levels they can tolerate to survive. The concentrations of drugs used were as follows: 40, 80, 120, 240, and 400 μg/ml for hygromycin B; 100, 200, 300, 600, and 1000 μg/ml for bleomycin; 5, 10, 15, 30, and 50 μg/ml for puromycin; and 200, 400, 600, 1,200, and 2,000 μg/ml for G418. Cells were inspected 8 days after drug selection.
PCR Analysis
Genomic DNA was isolated as previously described (1) with several modifications (15). Conventional PCR was performed as follows. Four sets of primers, Hyg-3S/ Hyg-RV, ble-S/ble-RV, puro-S/puro-3RV, and Ne-4S/ Ne-4RV, were used to identify the pcBIH, pcBIP, and pcBSN transgenes integrated into the genome of the STO transfectants. The oligonucleotide primers were as follows: the Hyg primer set (Hyg-3S, 5′-AAATCACG CCATGTAGTGTAT-3′; Hyg-RV, 5′-CGTCGCGGTGA GTTCAGGCTT-3′), which yields 442-bp fragments; the ble primer set (ble-S, 5′-ATGGCCAAGTTGACCAG TGC-3′; ble-RV, 5′-TCAGTCCTGCTCCTCGGCC-3′), which yields 375-bp fragments; the puro primer set (puro-S, 5′-GCAACCTCCCCTTCTACGAGC-3′; puro- 3RV, 5′-CGGGCGTCAGGCACCGGGCTT-3′), which yields 110-bp fragments; and the neo primer set (Ne-4S, 5′-CCAGCCGAACTGTTCGCCAGG-3′; Ne-4RV, 5′-TCAGAAGAACTCGTCAAGAAG-3′), which yields 297-bp fragments. As a negative control, 0.5 μg of genomic DNA from the untransfected STO cell was used. As a positive control, 5 ng of each plasmid was used.
The regular PCR amplification reactions were performed in a total volume of 10 μl containing 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.25 mM each of dATP, dCTP, dGTP, and dTTP, 1 mM each of sense and reverse primers, 2 μl of genomic DNA, and 0.5 U of rTaq polymerase (Takara Shuzo Co., Ltd., R001, Tokyo, Japan). PCR (40 cycles) was performed at 96°C for 10 s, 56°C for 1 min, and 72°C for 2 min. The PCR products (2 μl) were separated on a 2% agarose gel and visualized with ethidium bromide.
Results
After selection by selective drugs, eight SHB, nine SPB, and eight SNB clones were successfully isolated. The morphology of these cells, passaged over 18 generations, was indistinguishable from that of the parental STO cells (Fig. 2).
To test whether these isolated clones can support ES cells without affecting their undifferentiated property (i.e., ability to form moles), mouse ES cells were seeded onto each of the MMC-treated feeder clones. Five days after seeding, morphology of the ES cells was inspected under light microscopy. Three of eight (for SHB clones), three of nine (for SPB clones), and three of eight (for SNB clones) could support mouse ES cells without any overt morphological change (Fig. 3A). However, the other remaining clones failed to maintain the ES cells (Fig. 3B); in other words, differentiations of ES cells were frequently observed.
To examine the presence of transgenes in the transfected STO clones, genomic DNA isolated from SHB3 (carrying pcBIH), SPB3 (carrying pcBIP), and SNB2 (carrying pcBSN), all of which were proven to be good for maintenance of ES cells (Fig. 3A), were subjected to conventional PCR. As expected, each clone possessed two drug-resistant markers (Fig. 4).
Figure 4.
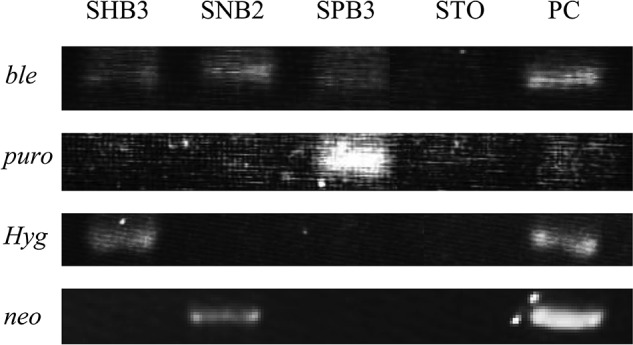
PCR analysis for the presence of selectable marker genes in stable transfectants (SHB3, SNB2, and SPB3) that are proven to support ES cells in undifferentiated state. STO indicates a parental cell used as negative control. PC indicates plasmids used as positive control. For detection of ble and puro, pcBIP was used. For detection of Hyg, pcBIH was used. For detection of neo, pcBSN was used.
Next, we performed sensitivity assay for SHB3, SPB3, and SNB2 clones, as described in Materials and Methods. The SHB2 clone survived in high concentrations of drugs (240 μg/ml of hygromycin B and 600 μg/ml of bleomycin). The SPB3 also survived in the presence of 30 μg/ml of puromycin and 300 μg/ml of bleomycin. The SNB2 clone survived in the presence of 400 μg/ml of G418 and 300 μg/ml of bleomycin. These results indicate that the SHB3, SPB3, and SNB2 clones have the ability to confer resistance to relatively high concentrations of selective drugs that would be enough to acquire gene-engineered ES/iPS cells.
Discussion
Besides MEFs and STO cells, human ES cells have been successfully maintained using several types of feeder cells that include human fetal muscle cells, adult fallopian tube-derived fibroblasts (13,14), fibroblasts differentiated from human ES cells (16,18,25), human placental fibroblasts (5,17), and adult bone marrow cells (4). In any case, it seems difficult to generate gene-engineered drugresistant feeder cells that support growth of genetically manipulated ES/iPS cells because of limited proliferation frequency like MEFs. The most ideal approach to generate drug-resistant feeder cells would be the immortalization of primary cultured cells like STO cells.
We found in this study that some drug-resistant STO clones failed to support mouse ES cell growth (as exemplified by SHB1, SPB4, and SNB1 in Fig. 3B). These cells were undistinguishable from those that successfully support the ES cell growth in view of cell morphology and proliferation rate (data not shown). The reason underlying such failure still remains dissolved. Gene transfection and subsequent drug selection may have affected the property of STO cell itself. On the other hand, it would be of interest to test gene expression pattern between these two types of cells using an expression microarray. From this study, genes involved in the support of the growth of ES/iPS cells in an undifferentiated state may be clarified.
Notably, SNL is a ready-made immortal MEF cell line that can support mouse ES cell growth (7). It expresses leukemia inhibitory factor (LIF) constitutively and is resistant to neomycin. In this context, it would be preferable to introduce a LIF expression unit to our present STO-derived gene-engineered cells. This attempt is now in progress. Furthermore, for convenience of researchers, generation of STO transfectants carrying multigenes such as neo, ble, puro, Hyg, and LIF would be desirable. This is also our future subject to be realized.
More importantly, testing the pluripotency of mouse ES cells maintained in our present gene-engineered cells (such as SHB3, SPB3, and SNB2 clones) is strictly required. This would be verified by chimeric mouse production through blastocyst injection of ES cells or by transplantation of ES cells underneath the skin of nude mice. Such trial is also being processed in our laboratory.
In conclusion, we generated the STO cell-derived SHB3, SPB3, and SNB2 clones that confer resistance to bleomycin/hygromycin B, bleomycin/puromycin, and bleomycin/neomycin, respectively. These cells would be suitable for acquisition of gene-engineered ES/iPS cells.
Acknowledgments
This work was supported in part by the Japanese Society for the Promotion of Science (no. 22890147). The authors declare no conflicts of interest.
References
- 1. Blin N.; Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 3(9):2303–2308;1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brook F. A.; Gardner R. L. The origin and efficient derivation of embryonic stem cells in the mouse. Proc. Natl. Acad. Sci. USA 94(11):5709–5712;1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen M.; Sun X.; Jiang R.; Shen W.; Zhong X.; Liu B.; Qi Y.; Huang B.; Xiang A. P.; Ge J. Role of MEF feeder cells in direct reprogramming of mousetail-tip fibroblasts. Cell Biol. Int. 33(12):1268–1273;2009. [DOI] [PubMed] [Google Scholar]
- 4. Cheng L.; Hammond H.; Ye Z.; Zhan X.; Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells 21(2):131–142;2003. [DOI] [PubMed] [Google Scholar]
- 5. Genbacev O.; Krtolica A.; Zdravkovic T.; Brunette E.; Powell S.; Nath A.; Caceres E.; McMaster M.; McDonagh S.; Li Y.; Mandalam R.; Lebkowski J.; Fisher S. J. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil. Steril. 83(5):1517–1529;2005. [DOI] [PubMed] [Google Scholar]
- 6. Martin G. R.; Evans M. J. Differentiation of clonal lines of teratocarcinoma cells: Formation of embryoid bodies in vitro. Proc. Natl. Acad. Sci. USA 72(4):1441–1445;1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMahon A. P.; Bradley A. The Wnt-1 (int-1) proto- oncogene is required for development of a large region of the mouse brain. Cell 62(6):1073–1085;1990. [DOI] [PubMed] [Google Scholar]
- 8. Nichols J.; Zevnik B.; Anastassiadis K.; Niwa H.; Klewe-Nebenius D.; Chambers I.; Scholer H.; Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95(3):379–391;1998. [DOI] [PubMed] [Google Scholar]
- 9. Osafune K.; Caron L.; Borowiak M.; Martinez R. J.; Fitz-Gerald C. S.; Sato Y.; Cowan C. A.; Chien K. R.; Melton D. A. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 26(3):313–315;2008. [DOI] [PubMed] [Google Scholar]
- 10. Park J. H.; Kim S. J.; Oh E. J.; Moon S. Y.; Roh S. I.; Kim C. G.; Yoon H. S. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol. Reprod. 69(6):2007–2014;2003. [DOI] [PubMed] [Google Scholar]
- 11. Pelletier J.; Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334(6180):320–325;1988. [DOI] [PubMed] [Google Scholar]
- 12. Reubinoff B. E.; Pera M. F.; Fong C. Y.; Trounson A.; Bongso A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 18(4):399–404;2000. [DOI] [PubMed] [Google Scholar]
- 13. Richards M.; Fong C. Y.; Chan W. K.; Wong P. C.; Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 20(9):933–936;2002. [DOI] [PubMed] [Google Scholar]
- 14. Richards M.; Tan S.; Fong C. Y.; Biswas A.; Chan W. K.; Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells 21(5):546–556;2003. [DOI] [PubMed] [Google Scholar]
- 15. Sato M.; Tada N. Preferential expression of osteocalcinrelated protein mRNA in gonadal tissues of male mice. Biochem. Biophys. Res. Commun. 215(1):412–421;1995. [DOI] [PubMed] [Google Scholar]
- 16. Saxena S.; Hanwate M.; Deb K.; Sharma V.; Totey S. FGF2 secreting human fibroblast feeder cells: A novel culture system for human embryonic stem cells. Mol. Reprod. Dev. 75(10):1523–1532;2008. [DOI] [PubMed] [Google Scholar]
- 17. Simon C.; Escobedo C.; Valbuena D.; Genbacev O.; Galan A.; Krtolica A.; Asensi A.; Sanchez E.; Esplugues J.; Fisher S.; Pellicer A. First derivation in Spain of human embryonic stem cell lines: Use of long-term cryopreserved embryos and animal-free conditions. Fertil. Steril. 83(1):246–249;2005. [DOI] [PubMed] [Google Scholar]
- 18. Stojkovic P.; Lako M.; Stewart R.; Przyborski S.; Armstrong L.; Evans J.; Murdoch A.; Strachan T.; Stojkovic M. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells 23(3):306–314;2005. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki T.; Seth A.; Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factormediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J. Biol. Chem. 283(6):3574– 3583;2008. [DOI] [PubMed] [Google Scholar]
- 20. Takahashi K.; Tanabe K.; Ohnuki M.; Narita M.; Ichisaka T.; Tomoda K.; Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872;2007. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi K.; Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676;2006. [DOI] [PubMed] [Google Scholar]
- 22. Talbot N. C.; Powell A. M.; Garrett W. M. Spontaneous differentiation of porcine and bovine embryonic stem cells (epiblast) into astrocytes or neurons. In Vitro Cell. Dev. Biol. Anim. 38(4):191–197;2002. [DOI] [PubMed] [Google Scholar]
- 23. Thomson J. A.; Itskovitz-Eldor J.; Shapiro S. S.; Waknitz M. A.; Swiergiel J. J.; Marshall V. S.; Jones J. M. Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147;1998. [DOI] [PubMed] [Google Scholar]
- 24. Ware L. M.; Axelrad A. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: Evidence that congenic mouse strains SIM and SIM.R differ at the Fv-1 locus. Virology 50(2):339–348;1972. [DOI] [PubMed] [Google Scholar]
- 25. Xu C.; Jiang J.; Sottile V.; McWhir J.; Lebkowski J.; Carpenter M. K. Immortalized fibroblast-like cells derived from human embryonic stem cells support undifferentiated cell growth. Stem Cells 22(6):972–980;2004. [DOI] [PubMed] [Google Scholar]
- 26. Yu J.; Vodyanik M. A.; Smuga-Otto K.; Antosiewicz-Bourget J.; Frane J. L.; Tian S.; Nie J.; Jonsdottir G. A.; Ruotti V.; Stewart R.; Slukvin I. I.; Thomson J. A. Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920;2007. [DOI] [PubMed] [Google Scholar]