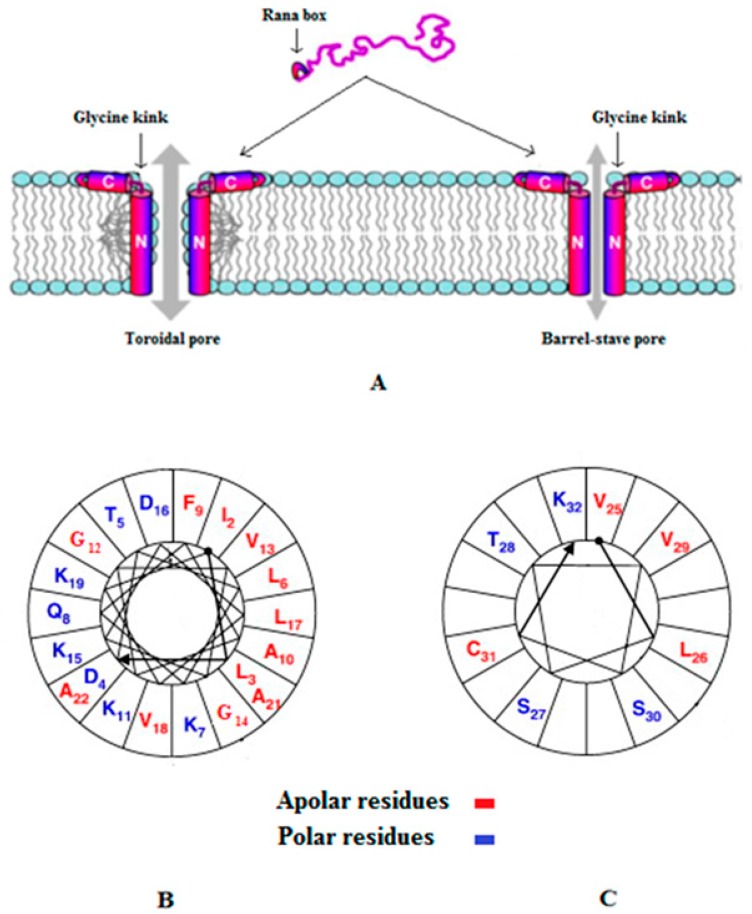
Figure 1.
Models for the membrane pore formation by E2EM. Figure 1 was revised from [115] and Figure 1A shows models for pore formation by E2EM, which are the toroidal pore and barrel stave mechanisms (Table 1) and are the best supported experimentally. In both models, the N-terminal 23 residues of the peptide spans the bilayer and a glycine kink orientates the 7 residue, C-terminal Rana box region of E2EM to lie parallel to the membrane surface. In this orientation, the Rana box region of the peptide, which is a cystein stabilized macrocyclic structure, interacts with the lipid head-group region of the membrane and stabilizes pore formation by E2EM [115]. The major difference between these models is that in the toroidal pore mechanism, the membrane leaflets deform to allow the lipid head-group region to remain in contact with the hydrophilic face of the E2EM membrane spanning region, which is not observed in the barrel stave mechanism [23]. For clarity, two monomers of E2EM are shown in the schematic pore above but oligomers formed by between five and ten peptide molecules have been proposed [115,120]. Similar models of membrane interaction appear to apply to the linear reduced form of the peptide [115], which is represented in our studies as E2EM-lin. Figure 1B,C show two-dimensional axial projections [126] for the membrane spanning region and Rana box domain of E2EM, respectively, that are involved in pore formation by the peptide. In both cases, these segments for amphipilic α-helices with wide hydrophobic faces that our data suggest would be maximized by alkaline pH, thereby promoting the potential for the mutual interaction of E2EM monomers and the formation of multimeric species involved in pore formation.