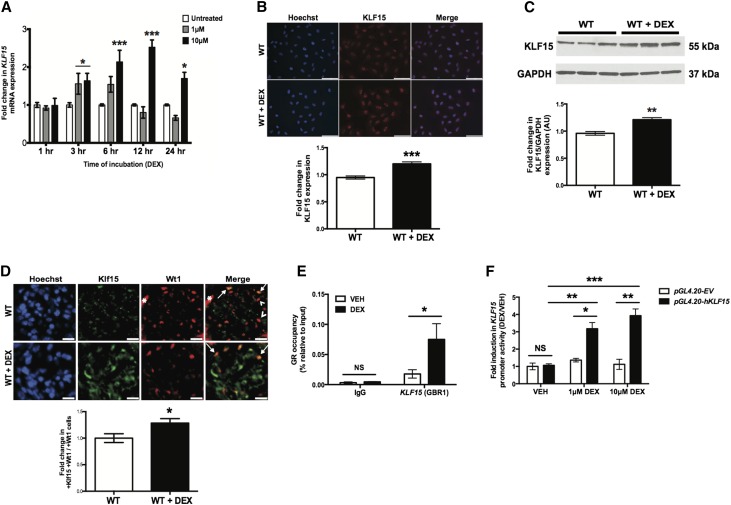
Figure 1.
KLF15 expression is increased with DEX treatment. Cultured human podocytes were initially differentiated for 14 days and subsequently treated with either DEX or vehicle (VEH) for 12 hours. RNA was extracted, and real-time PCR was performed. (A) KLF15 mRNA expression was compared between cultured human podocytes treated with and without DEX (n=6). *P<0.05; ***P<0.001 versus all groups (two–way ANOVA test with Tukey post-test). (B) Immunofluorescence staining for KLF15 with and without DEX for 12 hours is shown. The representative images of six independent experiments are shown in the upper panel. Magnification, ×20. In the lower panel, the intensity of KLF15 expression was quantified (n=6). ***P<0.001 (unpaired t test). (C) Protein was also extracted, and Western blot analysis for Klf15 was performed. The representative blot of three independent experiments is shown. The lower panel shows the quantification of Klf15 by densitometry (n=3). **P<0.01 (Mann–Whitney test). (D) This was confirmed by immunofluorescence using tissue from wild-type (WT) mice treated with and without DEX. The representative pictures of four mice in each group are shown in the upper panel. Arrows show colocalization of Klf15 and Wt1. Arrowheads show a lack of colocalization. Magnification, ×20. *Nonspecific Wt1 staining in the untreated WT mice. In the lower panel, in total, 30 glomeruli per mouse were selected, and quantification of Klf15 staining in the podocytes was determined by the ratio of Klf15+ and Wt1+ cells to Wt1+ cells (n=6). *P<0.05 (unpaired t test). (E) ChIP assay was performed to show the presence of GR binding in the promoter of KLF15 in differentiated human podocytes treated with DEX (10 μM) or VEH for 12 hours. IgG serves as control (n=6). *P<0.05 (Mann–Whitney test). (F) Human podocytes were transfected with reporter construct directed at the KLF15 promoter region (pGL4.20-hKLF15) or empty vector (pGL4.20-EV). Fold induction in KLF15 promoter activity is shown with DEX (1 or 10 μM) treatment compared with VEH-treated cells (n=6). *P<0.05; **P<0.01; ***P<0.001 (two–way ANOVA test with Tukey post-test).