Abstract
Distal tubular sodium retention is a potent driver of hypertension, and the thiazide–sensitive sodium-chloride cotransporter (NCC) has a key role in this process. In humans, factors regulating NCC are unclear, but in animal models, aldosterone is a potent regulator, possibly via effects on plasma potassium. We studied the effects of the mineralocorticoid fludrocortisone on the abundance of NCC and its phosphorylated form (pNCC) as well as WNK lysine deficient protein kinase 4 (WNK4) and STE20/SPS1–related, proline alanine–rich kinase (SPAK) in human urinary exosomes. We isolated exosomes from daily urine samples in 25 patients undergoing fludrocortisone suppression testing (100 μg every 6 hours for 4 days) to diagnose or exclude primary aldosteronism. Over the course of the test, NCC levels increased 3.68-fold (P<0.01) and pNCC levels increased 2.73-fold (P<0.01) relative to baseline. The ratio of pNCC/NCC dropped by 48% (P<0.01). The abundance of WNK4 increased 3.23-fold (P<0.01), but SPAK abundance did not change significantly (P=0.14). Plasma potassium concentration strongly and negatively correlated with pNCC, NCC, and WNK4 abundance (P<0.001 for all). This study shows that, in humans, mineralocorticoid administration is associated with a rapid increase in abundance of NCC and pNCC, possibly via the WNK pathway. These effects may be driven by changes in plasma potassium.
Keywords: NCC, aldosterone, Na transport, Exosomes
Sodium handling in the distal renal tubule is an important contributor to BP regulation and a valuable target for antihypertensive drugs. The thiazide-sensitive Sodium-chloride cotransporter (NCC) is responsible for reabsorbing 5%–10% of the filtered sodium load, and its importance is clear from not only the efficacy of thiazide diuretics but also, the monogenetic disorders of Gitelmans and Gordon syndromes, where NCC dysregulation causes profound BP derangement.1 The identification of the genetic causes of Gordon syndrome has revealed a complex system of kinases that control NCC trafficking and phosphorylation (increases transporter activity). Current models propose the with no lysine kinases (WNKs) WNK4 and WNK1 to be critical regulators of NCC abundance and phosphorylation, with STE20/SPS1–related, proline alanine–rich kinase (SPAK) also involved.2 Most studies on which these models are based have been performed in animals or cells and resulted in uncertainty regarding the role of some proteins (e.g., WNK4 has been shown as both a positive and a negative regulator under different experimental conditions).3–6 Animal models have suggested that the renin-angiotensin-aldosterone system is important for NCC regulation, with both aldosterone and angiotensin II able to increase abundance and phosphorylation of NCC.7–9 The recognition of a perhaps dominant role for potassium in NCC regulation has also recently emerged.10 The role of the renin-angiotensin-aldosterone system and potassium in the regulation of NCC and the WNK-SPAK pathway in humans is, however, less clear, although the abundance of phosphorylated NCC (pNCC) has been reported to be increased in patients with primary aldosteronism (PA) compared with essential hypertensives, with possible diagnostic implications.11
The study of tubular physiology in vivo has been advanced by techniques to isolate urinary exosomes.12 These are released by the renal tubular epithelium and contain constituents of their cells of origin, allowing an indirect measurement of the cellular abundance of various proteins.13 Importantly, this technique has been applied to measure expression and post-translational modification of a number of channels and transporters, including NCC.14–16
In this study, we analyzed urinary exosomes isolated from humans after mineralocorticoid administration. The aim was to examine the actions of mineralocorticoids on NCC abundance and phosphorylation as well as effects on the intermediary kinases WNK4 and SPAK.
We obtained daily morning urine samples from 26 fludrocortisone suppression tests (FST) performed in 25 individuals. Eight subjects were studied postadrenalectomy for aldosterone-producing adenoma (one patient was studied twice—both before and after surgery). Sixteen of the patients were men, and nine were women, with a mean age of 55.8 years old, a mean BP of 141/84 mmHg, and a median of two antihypertensive medications (additional patient characteristics are in Supplemental Table 1). Twenty of the patients had positive fludrocortisone suppression tests, thereby confirming PA (two postoperative patients had residual unsuppressible aldosterone production).
We used ALG-2–interacting protein X (ALIX) as a positive control for exosome isolation, and ALIX abundance increased modestly (median of 1.28-fold) but significantly over the test (P<0.001), suggesting a possible increase in exosome excretion with fludrocortisone (Figure 1, Tables 1–5). We additionally analyzed tumor susceptibility gene 101 (TSG101) as an additional exosomal marker in a subset of samples comprising 19 of 26 of the cohort, finding again a modest but consistent increase in abundance (median of 1.14-fold) from day 0 to day 4 (P=0.02). We, therefore, controlled for changes in the relative abundance of ALIX in all subsequent multivariate modeling when assessing for significance in changes in abundance of the target proteins.
Figure 1.
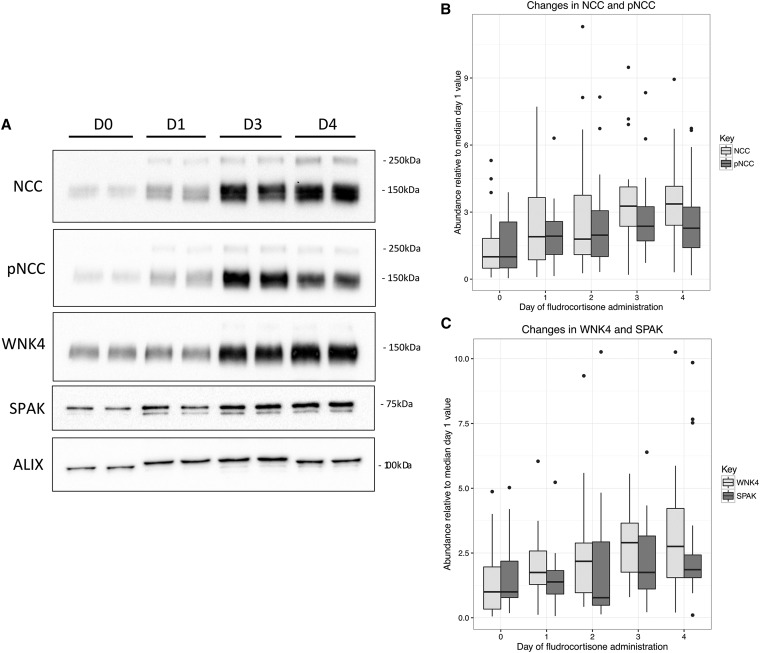
Increases in NCC, pNCC and WNK4 during FST. (A) Representative immunoblot from a single patient of basal day (D0), D1, D3, and D4 (additional summary blots available in Supplemental Appendix). (B) Box plot of NCC and pNCC levels by day of fludrocortisone suppression test. The upper and lower hinges correspond to the first and third quartiles, and the whiskers extend to the highest and lowest values within 1.5× the interquartile range, with outliers indicated by dots. Levels are measured as OD per milligram of creatinine in the sample (not adjusted for changes in ALIX), where results have been standardized to the respective median day 0 (prefludrocortisone) values. (C) Box plot of WNK4 and SPAK levels by day of fludrocortisone suppression test. The upper and lower hinges correspond to the first and third quartiles, and the whiskers extend to the highest and lowest values within 1.5× the interquartile range, with outliers indicated by dots. Levels measured as OD per milligram of creatinine in the sample (not adjusted for changes in ALIX), where results have been standardized to the respective median day 0 (prefludrocortisone) values.
Table 1.
Changes in NCC abundance
| Day of FST | NCCa | Fold Change Relative to Day 1 | P Value (Relative to Day 1) | P Value (Trend) |
|---|---|---|---|---|
| 0 | 3.20 | 1 | ||
| 1 | 6.22 | 1.94 | 0.07 | |
| 2 | 5.87 | 1.83 | 0.05 | <0.001 |
| 3 | 10.70 | 3.34 | 0.001 | |
| 4 | 11.76 | 3.68 | 0.001 |
P values are for multivariate models controlling for changes in ALIX (except in the ALIX model) as well as cure of PA, dose of potassium supplementation, and patient weight.
Expressed in units of OD per milligram of creatinine.
Table 5.
Changes in ALIX abundance
| Day of FST | ALIXa | Fold Change Relative to Day 1 | P Value (Relative to Day 1) | P Value (Trend) |
|---|---|---|---|---|
| 0 | 2.51 | 1 | ||
| 1 | 2.54 | 1.01 | 0.22 | |
| 2 | 2.63 | 1.05 | 0.07 | 0.001 |
| 3 | 2.79 | 1.11 | 0.03 | |
| 4 | 3.21 | 1.28 | 0.001 |
P values are for multivariate models controlling for changes in ALIX (except in the ALIX model) as well as cure of PA, dose of potassium supplementation, and patient weight.
Expressed in units of OD per milligram of creatinine.
We observed a >3.5-fold increase in median NCC abundance over the test (Figure 1B, Table 1) (P<0.001 for univariate association). In univariate modeling, cure of PA (P=0.03) and dose of potassium supplementation (P<0.001) were additional significant negative correlates, but in multivariate modeling, only the association with day of FST (P<0.001) and cure of PA (P=0.03) remained. We also observed a >2.5-fold increase in median pNCC abundance (Figure 1B, Table 2) (P<0.001 for univariate association). In multivariate modeling, day of FST remained significant (P<0.001), but additionally, cure of PA (P=0.03) and dose of potassium (P=0.01) were significant negative correlates, and patient weight was a significant positive correlate (P=0.003).
Table 2.
Changes in pNCC abundance
| Day of FST | pNCCa | Fold Change Relative to Day 1 | P Value (Relative to Day 1) | P Value (Trend) |
|---|---|---|---|---|
| 0 | 4.83 | 1 | ||
| 1 | 9.60 | 1.99 | 0.08 | |
| 2 | 9.83 | 2.04 | 0.07 | <0.001 |
| 3 | 11.81 | 2.45 | 0.001 | |
| 4 | 13.20 | 2.73 | <0.001 |
P values are for multivariate models controlling for changes in ALIX (except in the ALIX model) as well as cure of PA, dose of potassium supplementation, and patient weight.
Expressed in units of OD per milligram of creatinine.
For WNK4, we found a 3.2-fold increase over the test (Figure 1C, Table 3) (P<0.001 for univariate association). In multivariate modeling, day of FST (P<0.001) and patient weight (P=0.002) were significant correlates, and cure of PA (P=0.002) and dose of potassium supplement (P=0.04) were significant negative correlates. We also found an increase of 2.2-fold in median SPAK abundance (Figure 1C, Table 4) (P=0.004 for univariate association), but after adjusting for changes in relative ALIX abundance, this was no longer significant (P=0.14).
Table 3.
Changes in WNK4 abundance
| Day of FST | WNK4a | Fold Change Relative to Day 1 | P Value (Relative to Day 1) | P Value (Trend) |
|---|---|---|---|---|
| 0 | 4.30 | 1 | ||
| 1 | 7.84 | 1.82 | 0.14 | |
| 2 | 9.77 | 2.27 | 0.17 | <0.001 |
| 3 | 13.00 | 3.02 | 0.003 | |
| 4 | 14.09 | 3.23 | <0.001 |
P values are for multivariate models controlling for changes in ALIX (except in the ALIX model) as well as cure of PA, dose of potassium supplementation, and patient weight.
Expressed in units of OD per milligram of creatinine.
Table 4.
Changes in SPAK abundance
| Day of FST | SPAKa | Fold Change Relative to Day 1 | P Value (Relative to Day 1) | P Value (Trend) |
|---|---|---|---|---|
| 0 | 1.80 | 1 | ||
| 1 | 2.49 | 1.38 | 0.56 | |
| 2 | 1.39 | 0.77 | 0.84 | 0.14 |
| 3 | 3.15 | 1.75 | 0.65 | |
| 4 | 3.88 | 2.16 | 0.18 |
P values are for multivariate models controlling for changes in ALIX (except in the ALIX model) as well as cure of PA, dose of potassium supplementation, and patient weight.
Expressed in units of OD per milligram of creatinine.
The average pNCC-to-NCC ratio almost halved within the first 24 hours of fludrocortisone administration and thereafter, remained approximately static (P=0.001; ratio log transformed for modeling) (Supplemental Figure 1,Table 6).
Table 6.
pNCC-to-NCC ratio
| Day of FST | pNCC-to-NCC Ratioa | Fold Change Relative to Day 1 | P Value (Relative to Day 1)b | P Value (Trend)b |
|---|---|---|---|---|
| 0 | 2.17 | 1 | ||
| 1 | 1.17 | 0.54 | 0.23 | |
| 2 | 1.19 | 0.55 | 0.02 | <0.001 |
| 3 | 1.10 | 0.51 | 0.003 | |
| 4 | 1.13 | 0.52 | 0.002 |
Median.
For modeling, the pNCC-to-NCC ratio was log transformed.
Eight patients were studied postadrenalectomy, two of whom were confirmed to have residual unsuppressible aldosterone production (one patient was studied both pre- and postoperatively). Day 0 potassium levels (3.6 versus 4.7 mmol/L; P=0.003) and renin levels (6 versus 19 mU/L; P=0.004) were lower in patients with PA. Day 0 aldosterone levels (643 versus 144 nmol/L; P<0.001) and the aldosterone-to-renin ratio were higher (133 versus 12.9; P<0.001) in patients with PA. The median day 4 aldosterone level in those with PA was 657.5 nmol/L compared with 71 nmol/L in those in whom PA was excluded (P<0.001). In a standardized comparison of relative day 0 abundance (corrected for ALIX), the median relative abundance of NCC was 4.04-fold higher in patients with confirmed PA compared with those without (P<0.01) as was the relative abundance of pNCC (5.77-fold higher; P=0.03) and WNK4 (6.12-fold higher; P<0.01). Relative SPAK abundance was not significantly different (median of 0.58-fold lower in patients with PA; P=0.35) (Supplemental Figure 2).
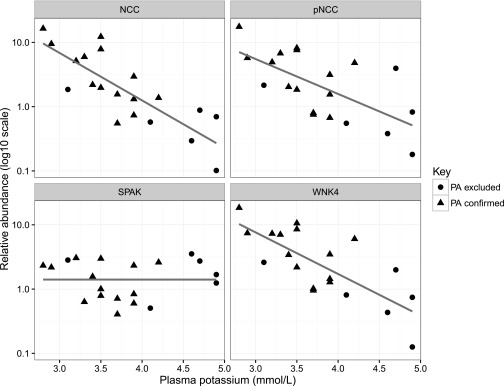
During FST, urinary potassium increased as did potassium supplementation, but plasma potassium remained stable over the test (detailed in Supplemental Table 2). For the following models, the relative abundances of NCC, pNCC, WNK4, and SPAK were log10 transformed to obtain Gaussian distributions. At day 0 (before fludrocortisone or potassium supplementation initiation), there was a strong inverse correlation between the relative NCC abundance (ALIX corrected) and plasma potassium levels (R2=0.66; P<0.001). There was also a significant negative correlation between plasma potassium and pNCC (R2=0.43; P=0.002) and WNK4 (R2=0.57; P<0.001) but not SPAK (Figure 2).
Figure 2.
Correlations with plasma potassium at baseline (pre-fludrocortisone). Relative abundance of pNCC, WNK4, and SPAK (normalized to control sample across all blots and ALIX abundance) at baseline before administration of fludrocortisone versus plasma potassium. Plotted on log10 y axis with linear regression lines.
Positive correlations were also present between aldosterone and relative NCC abundance (R2=0.34; P<0.01) and relative WNK4 abundance (R2=0.24; P=0.03), but a positive correlation between aldosterone and relative pNCC abundance (R2=0.17; P=0.07) was not statistically significant (Supplemental Figure 3).
The mean serum sodium at the start of the test was 139 mmol/L, with a mean creatinine of 75 μmol/L. Median plasma renin levels were 6 mU/L at baseline and 3.5 mU/L on day 4 (P=0.05), and 24-hour urinary sodium levels were 146 mmol/d (median) at baseline and 208.5 mmol/d on day 4 (P=0.05).
This study showed that mineralocorticoid administration in humans was associated with a rapid and sustained increase in the levels of NCC and pNCC in urinary exosomes. NCC levels increased more than pNCC levels, resulting in a decreased abundance ratio between these two forms of NCC. These changes in NCC were accompanied by increased WNK4. Although there was also a trend toward an increase in SPAK, this did not reach statistical significance. We also found significant negative associations between plasma potassium level and abundance of NCC, pNCC, and WNK4 at baseline and weaker positive associations between aldosterone and abundance of these proteins. Patients with PA had a greater abundance of NCC, pNCC, and WNK4 than those without both before and during mineralocorticoid administration.
The effects of aldosterone on distal tubular salt handling have historically been attributed to ENaC, but more recent animal and cell model–based evidence has also suggested a role in NCC regulation. Aldosterone infusion in rats increases pNCC and NCC abundance,7,17 and angiotensin II infusion seems to have similar effects.8,17 Furthermore, aldosterone and angiotensin II can act independently or additively.17,18 The exact pathways by which aldosterone and angiotensin II affect NCC are not entirely clear, although studies suggest that this may be mediated by the WNK-SPAK pathway. For example, angiotensin II infusion increased SPAK in the cortex of the rat kidney accompanied by increased pNCC and NCC, but these increases apparently occurred without alterations in WNK4.8,17 In other models, however, angiotensin II was dependent on WNK4 to regulate NCC.19 Furthermore, aldosterone increases SPAK expression, but effects on WNK4 have been inconsistent.17,18 The apparent inconsistency of effects of aldosterone on WNK4 may be attributable to WNK4 acting as a molecular switch with the capacity to activate or inhibit NCC depending on the physiologic state of the organism,20 a process critically dependent on intracellular chloride concentration.21,22
This is the first study in humans to clearly document a relationship between mineralocorticoids and NCC, pNCC, and WNK4 abundance. These findings are consistent with a model in which mineralocorticoids increase NCC expression and phosphorylation via positive regulation of the WNK4 pathway.
Recent evidence has also suggested an important and perhaps dominant role for potassium in regulating NCC, possibly by chloride-driven suppression of WNK4 activity.23 In fact, a paradigm shift in understanding of the apparent link between NCC and mineralocorticoids is currently evolving, with a number of recent animal studies suggesting that the influence of aldosterone on NCC is primarily driven by the concurrent changes in plasma potassium rather than direct actions of the mineralocorticoid receptor in the DCT.10,21,24 Supporting this concept and similar to animal studies, we found a strong negative correlation between plasma potassium and NCC and pNCC abundance as well as WNK4 at baseline, which was stronger than the relationship with aldosterone.23 During FST, however, we found that NCC, pNCC, and WNK4 increased with mineralocorticoids, despite no appreciable change in plasma potassium, although the dose of supplemental potassium was a significant independent negative correlate with abundance of pNCC and WNK4. In this context, plasma potassium may have been too insensitive of a measure relative to dietary input, or it may be that the sample number was too small to detect differences. Overall, we cannot determine the precise mechanism by which mineralocorticoids apparently influence NCC in this study. It is certainly possible, and perhaps more likely (given the animal evidence) that, rather than a direct action of mineralocorticoid signaling on NCC, the changes that we observed were driven by a secondary physiologic stimulus, such as the levels of potassium. Additional studies of this interesting and potentially clinically important link to potassium are warranted, with a particular need for studies in humans given the significant difference in dietary potassium intake between mice and humans.25
The increasingly apparent relationship between aldosterone, potassium, and NCC has important implications for our understanding of hypertension pathophysiology and also, treatment strategies. For example, spironolactone is now recognized as a potent antihypertensive agent, particularly in the setting of resistant hypertension, where fluid and sodium retention is of great importance.26,27 It is likely that this potency is caused by not only reducing expression of ENaC but also, reducing the abundance and phosphorylation of NCC, effects suggested in some animal models.28 Furthermore, this study extends the findings of increased exosomal pNCC in hypertensive patients with PA by Van der Lubbe et al.,11 also showing increased NCC and WNK4 in such patients compared with those without PA. Whether this has diagnostic utility or not would require a prospective study with larger patient numbers during the diagnostic workup.
This study is not without limitations. Because patients were studied during fludrocortisone suppression testing, we cannot rule out a confounding effect from sodium loading. Sodium levels, however, did not change significantly during the test, despite an increase in both intake and excretion. It is also possible that the doses of fludrocortisone used could have a mild glucocorticoid activity. The majority of the patients had confirmed PA, and we are limited by not having a true normal control. Although our control group, the six patients cured of aldosteronism, had normal aldosterone, renin, and potassium levels at baseline, it could be argued that these patients were not truly normal, because they had only a single adrenal gland. Additionally, we cannot be certain about other aspects of the WNK pathway, particularly WNK1, which has recently been shown to be important for NCC activation.29 We can only draw limited conclusions regarding SPAK given that we were unable to measure phosphorylated SPAK/OSR1 in this study, which is undoubtedly of greater physiologic importance.
In summary, mineralocorticoid administration was associated with an increase in NCC abundance and phosphorylation in humans, and we also found evidence for a strong negative link with plasma potassium. These effects may be mediated via WNK4. These findings highlight the importance of aldosterone and potassium to sodium balance in the distal tubule and hence, BP regulation in humans.
Concise Methods
Participants
Patients undergoing fludrocortisone suppression testing to exclude or confirm the diagnosis of PA at the Princess Alexandra Hospital were recruited to participate. All participants gave informed written consent, and the project was approved by the Metro South Human Research Ethics Committee.
Fludrocortisone Suppression Test
Medications known to affect plasma renin and aldosterone levels were stopped for at least 4 weeks before the test (6 weeks in the case of diuretics), and hypertension was controlled with verapamil slow release plus or minus hydralazine and prazosin. During the 4-day test, patients were administered fludrocortisone at 100 μg every 6 hours with a high-sodium diet supplemented by 3×600 mg slow sodium tablets given thrice daily with meals and sufficient oral slow–release potassium chloride to maintain normokalaemia.
Urinary Exosome Isolation and Analyses
An approximately 50-ml second morning urine sample was collected daily between 9:00 and 10:00 ante meridian (1–2 hours after breakfast in all patients) and treated with protease inhibitor cocktail (cOmplete EDTA-Free; Roche, Basel, Switzerland) before freezing at −80°C. The timing of the urine sample was chosen to fit in with the timing of the fludrocortisone suppression test and because preliminary testing had shown the highest levels of NCC expression in the morning (data not shown). The urine sample on day 1 was collected before fludrocortisone and slow sodium administration and therefore, regarded as a baseline. Urinary exosome isolation was performed using a variation of a technique described previously by Pisitkun et al.12 Briefly, urine samples were thawed and vigorously vortexed before aliquoting a 48-ml sample for analysis. The samples were subjected to 17,000×g centrifugation (Avanti J-30I; Beckman Coulter, Inc., Brea, CA) at 4°C for 15 minutes to eliminate whole cells and debris, and the supernatant was then incubated with 1 ml 200 mg/ml dithiothreitol (V3155; Promega, Madison, WI) per 24-ml sample for 10 minutes at 37°C. The samples then underwent ultracentrifugation at 200,000×g (Optima L-100XP; Beckman Coulter, Inc.) for 60 minutes at 4°C, and the resulting pellet was then resuspended in 4 ml PBS with another 100 μl 200 mg/ml dithiothreitol before further ultracentrifugation at 200,000×g at 4°C for 60 minutes. The pellet was resuspended in 110 μl 0.1% SDS, and protein concentration was measured by spectrophotometer (Thermo Scientific Nanodrop Lite; Thermo Scientific). Samples were then treated with 5× laemmli buffer (1:4 volume) and incubated at 60°C for 10 minutes before freezing at −80°C until use.
Immunoblotting
SDS-PAGE was carried out with 8% polyacrylamide minigels, and proteins were transferred to polyvinyl difluoride membranes (Bio-Rad, Hercules, CA) under a constant current of 300 mA for 3 hours at 4°C. After blocking with 3% BSA (A3858; Sigma-Aldrich, St. Louis, MO) in TBS, membranes were probed with affinity–purified polyclonal antibody to NCC (1/1000; AB3553; Merck Millipore), pNCC (1/1000),30 WNK4 (1/1000; ab126477; Abcam, Inc., Cambridge, MA), ALIX (1/1000; ABC40; Merck Millipore), or an mAb to SPAK (1/1000; ab128894; Abcam, Inc.) or TSG101 (1/1000; ABC649; Merck Millipore). NCC was measured as the single dominant band at approximately 150 kD, pNCC also was measured as the single dominant band at approximately 150 kD, WNK4 was measured as the single dominant band at 150 kD, the dominant bands of SPAK were measured at 75 kD, the dominant bands of ALIX were measured at 96 kD, and the dominant bands of TSG101 were measured at 45 kD. The secondary antibody was goat anti–rabbit IgG antibody, HRP conjugate (12–348; Merck Millipore) used in a 1/20,000 dilution. Sites of antibody-antigen reaction were visualized by luminol–based enhanced chemiluminescence (1705061; Bio-Rad) before exposure by Bio-Rad ChemiDoc XRS+ Imager with Image Lab software. Relative quantitation of the band densities from immunoblots was carried out with ImageJ software. ALIX was used as a positive control confirming isolation of exosomes, and creatinine was used to normalize samples, because it correlated well with both ALIX abundance and apparent urine concentration. Creatinine concentration was measured using a colorimetric assay (ab65340/; Abcam, Inc.; MAK080; Sigma-Aldrich). OD was measured by a Multiskan(tm) GO Microplate Spectrophotometer (Thermo Scientific) at OD=570 nm.
Statistical Methods
For the main analysis, we used creatinine-corrected NCC, pNCC, WNK4, and SPAK levels as dependent variables, with day of FST being the primary independent variable. Day of FST (as a fixed effect) was modeled separately as a continuous variable to determine the overall trend of the effect of cumulative exposure to fludrocortisone on the abundance of the target proteins and separately as a categorical variable to determine significance in change compared with baseline by day. The main analysis used a longitudinal design with repeated observations in the same individuals over time. For each dependent variable, a random effect model was used that included all data from all time periods simultaneously, with observations over time from the same patient sharing the same random effects, assuming different random effects for different patients. For multivariate models, relative ALIX abundance was included as a covariate in all models to account for a possible increase in exosome excretion during the test. We also assessed aldosterone levels, renin levels, sex, weight, cure or confirmation of PA, plasma potassium level, the dose of supplemental potassium, and the doses of hydralazine and prazosin as potential covariates using a backward process of elimination to arrive at the final models. To assess baseline correlations, we used simple linear models, log transforming skewed variables to achieve approximately normal distributions. To assess baseline relative abundance across multiple Western blots, we normalized to a common standard (single normal volunteer sample) included on each blot as well as to ALIX abundance. Statistical significance was assessed at a level of P<0.05. P values for group comparisons were calculated using the t test or the Mann–Whitney test for nonparametric distributions and the fixed effects from the mixed effects models (all repeated measures analysis) using the lmerTest package, which implements the Satterthwaite approximation.31 We used R (R Foundation for Statistical Computing), version 3.2.1.
Disclosures
None.
Supplementary Material
Acknowledgments
M.J.W. is supported by a postgraduate scholarship from the Princess Alexandra Hospital Research Foundation, and this work was also supported by a project grant from the Princess Alexandra Hospital Research Foundation. Funding to R.A.F. is provided by Danish Council for Independent Research, Natural Sciences project DFF–4002-00364 and the Lundbeck Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015111221/-/DCSupplemental.
References
- 1.Lifton RP, Gharavi AG, Geller DS: Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 2.O’Shaughnessy KM: Gordon Syndrome: A continuing story. Pediatr Nephrol 30: 1903–1908, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP: Molecular pathogenesis of inherited hypertension with hyperkalemia: The Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci U S A 100: 680–684, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CL, Angell J, Mitchell R, Ellison DH: WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golbang AP, Murthy M, Hamad A, Liu CH, Cope G, Van’t Hoff W, Cuthbert A, O’Shaughnessy KM: A new kindred with pseudohypoaldosteronism type II and a novel mutation (564D>H) in the acidic motif of the WNK4 gene. Hypertension 46: 295–300, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S: Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Reports 3: 858–868, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen MT, Lee DH, Delpire E, McDonough AA: Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: Distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velázquez H, Bartiss A, Bernstein P, Ellison DH: Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol 270: F211–F219, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH: Direct and indirect mineralocorticoid effects determine distal salt transport [published online ahead of print December 28, 2015]. J Am Soc Nephrol doi:ASN.2015070815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Lubbe N, Jansen PM, Salih M, Fenton RA, van den Meiracker AH, Danser AH, Zietse R, Hoorn EJ: The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension 60: 741–748, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101: 13368–13373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salih M, Zietse R, Hoorn EJ: Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Mayan H, Attar-Herzberg D, Shaharabany M, Holtzman EJ, Farfel Z: Increased urinary Na-Cl cotransporter protein in familial hyperkalaemia and hypertension. Nephrol Dial Transplant 23: 492–496, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Corbetta S, Raimondo F, Tedeschi S, Syrèn ML, Rebora P, Savoia A, Baldi L, Bettinelli A, Pitto M: Urinary exosomes in the diagnosis of Gitelman and Bartter syndromes. Nephrol Dial Transplant 30: 621–630, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Huebner AR, Cheng L, Somparn P, Knepper MA, Fenton RA, Pisitkun T: Deubiquitylation of protein cargo is not an essential step in exosome formation. Mol Cell Proteomics 15: 1556–1571, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ: Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011 [DOI] [PubMed] [Google Scholar]
- 18.van der Lubbe N, Lim CH, Meima ME, van Veghel R, Rosenbaek LL, Mutig K, Danser AH, Fenton RA, Zietse R, Hoorn EJ: Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. Pflugers Arch 463: 853–863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G: Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci U S A 106: 4384–4389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazúa-Valenti S, Gamba G: Revisiting the NaCl cotransporter regulation by with-no-lysine kinases. Am J Physiol Cell Physiol 308: C779–C791, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH: KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A 111: 11864–11869, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J: The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflugers Arch 468: 849–858, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Oxlund CS, Henriksen JE, Tarnow L, Schousboe K, Gram J, Jacobsen IA: Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: A double blind randomized clinical trial. J Hypertens 31: 2094–2102, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Egan BM, Li J: Role of aldosterone blockade in resistant hypertension. Semin Nephrol 34: 273–284, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S: Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Roy A, Al-Qusairi L, Donnelly BF, Ronzaud C, Marciszyn AL, Gong F, Chang YP, Butterworth MB, Pastor-Soler NM, Hallows KR, Staub O, Subramanya AR: Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action. J Clin Invest 125: 3433–3448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA: Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsova A, Brockhoff PB, Christensen RHB: lmerTest: Tests in Linear Mixed Effects Models. R Package Version 2.0-29, 2015. Available at: https://cran.r-project.org/. Accessed October 19, 2015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.