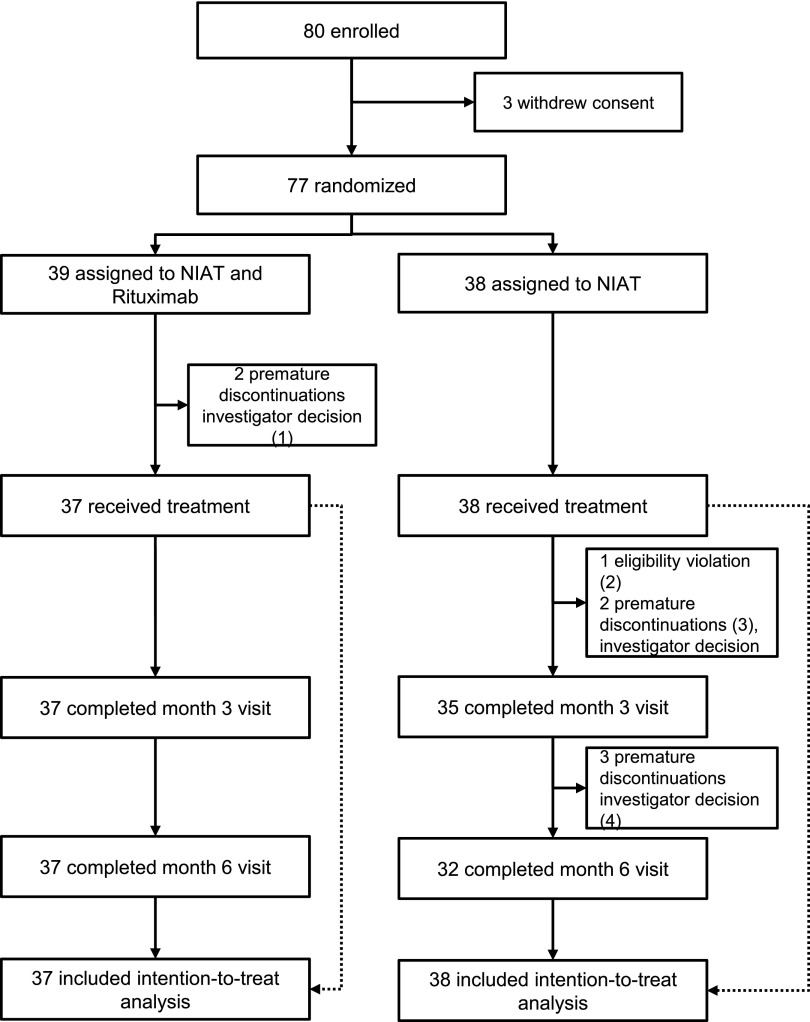
Figure 1.
Flow chart of the trial. The flow chart shows that premature discontinuation occurred in five patients within 3 months after inclusion: (1) two remissions at day 1 or inclusion; (2) one in NIAT for <6 months; (3) one lost to follow-up and one diagnosed with a pulmonary neoplasia; and (4) three treatment shifts between 3 and 6 months: two received rituximab or steroids because of deterioration of clinical condition and one was referred to another center.