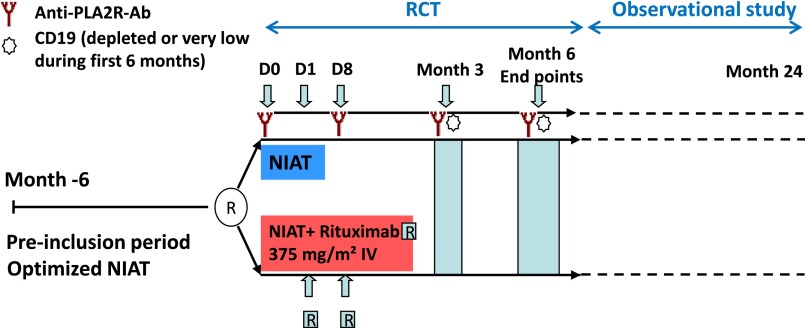
Figure 3.
Study design. After a pre-inclusion period of 6 months during which NIAT was optimized, patients were assigned either to NIAT plus Rituximab (375 mg/m2, two infusions at days 1 and 8) or to NIAT alone. Serum for anti-PLA2R-Ab determination was sampled at days 0 and 8, months 3 and 6, CD19 counts were determined at months 3 and 6; end points were assessed at month 6. The RCT was followed by an observational study during which follow-up was extended up to 24 months. IV, intravenous; R, rituximab.