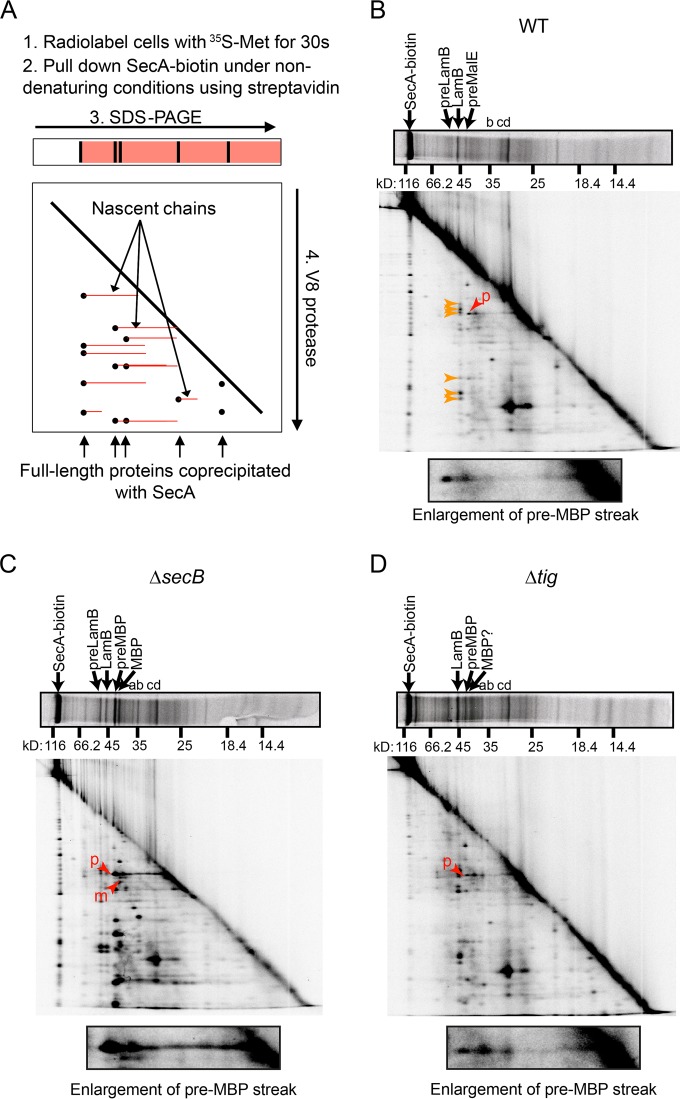
FIG 2.
2-D gel analysis of the interaction of SecA-biotin with nascent polypeptide in vivo. (A) Diagrammatic representation of 2-D gel analysis. Cells expressing SecA-biotin as their sole copy of SecA were grown in M63 maltose containing IPTG and radiolabeled for 30 s with 35S-methionine, and SecA-biotin was pulled down from the cell lysates under nondenaturing conditions. The proteins that coprecipitated with SecA-biotin were resolved in the first dimension using SDS-PAGE. Gel slices containing the resolved proteins were then loaded on to a second gel and subjected to in-gel proteolysis using the V8 protease. Proteolytic fragments of full-length proteins (black) resolve as spots running below the full-length bands, while nascent polypeptides (red) containing the same proteolytic fragments resolve as streaks extending back from these spots in the second dimension. (B to D) 2-D gel analysis was carried out on cells expressing SecA-biotin as the sole copy of SecA (DRH839) (B) and on ΔsecB (DRH841) (C) and Δtig (DRH866) (D) mutants of DRH839. The running positions of full-length precursor LamB (preLamB), mature LamB, pre-MBP, and mature MBP are indicated in the first dimension. Full-length bands with molecular masses corresponding to precursor OmpC (a), mature OmpC (b), precursor OmpA (c), and mature OmpA (d) are indicated. Red arrowheads in the second dimension indicate the N-terminal peptide fragments of preMBP (p) and mature MBP (m). Orange arrowheads indicate the peptide fragments generated by full-length LamB. Question marks indicate that identification of the full-length species or peptide fragment could not be made unambiguously due to lack of signal. An enlargement of the region of the gel corresponding to the N-terminal proteolytic fragment of precursor-length MBP is depicted below each 2-D gel.