FIG 5.
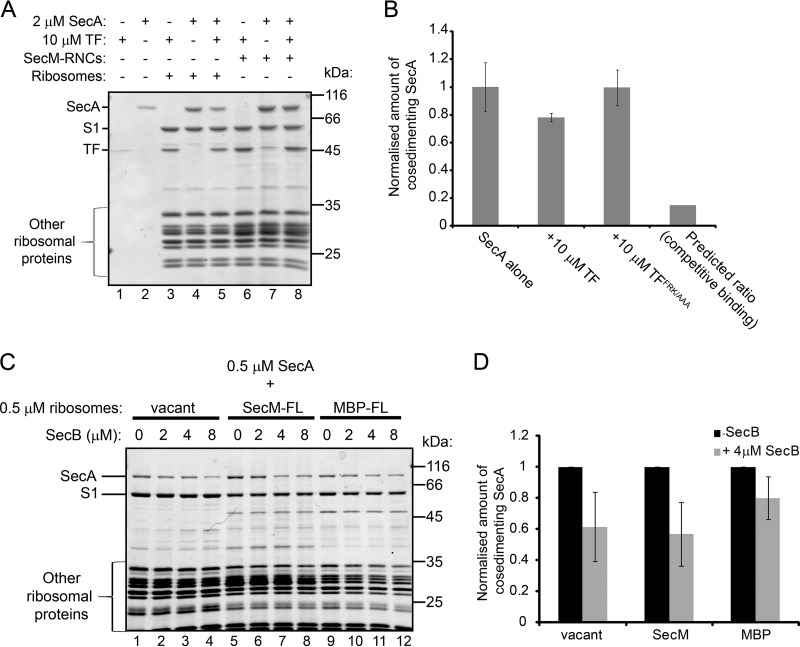
Effects of the presence of TF and SecB on the interaction of ribosome binding by SecA. (A) Vacant 70S ribosomes (1 μM) or SecM-RNCs (1 μM) were incubated with SecA (2 μM) or TF (10 μM) or both, as indicated. After equilibration, binding reaction mixtures were layered on a 30% sucrose cushion and centrifuged at >200,000 × g. The ribosomal pellet fractions were resolved by SDS-PAGE and visualized by Coomassie staining. (B) SecA (2 μM) was incubated with vacant 70S ribosomes (1 μM) in the absence or presence of wild-type TF (10 μM) or a ribosome binding-deficient variant, TFRFK/AAA (10 μM). After equilibration, binding reaction mixtures were layered on a 30% sucrose cushion and centrifuged at >200,000 × g, and the amount of SecA in the ribosomal pellet relative to the amount of ribosomal protein L1 was determined by quantitative Western blotting. Confidence intervals shown by error bars represent 1 standard deviation from the average of three independent experiments. The predicted ratio is the amount of SecA predicted to cosediment with ribosome in the presence of TF if binding were fully competitive. (C) SecA (0.5 μM) was incubated with vacant 70S ribosomes (0.5 μM), RNCs containing full-length SecM (0.5 μM) or MBP (SecM-FL and MBP-FL, respectively) (0.5 μM), or MBP-FL RNCs (0.5 μM). After equilibration, binding reaction mixtures were layered on a 30% sucrose cushion and centrifuged at >200,000 × g. The ribosomal pellet fractions were resolved by SDS-PAGE and visualized by Coomassie staining. (D) Quantitation of the results shown in panel C. The amounts of SecA that cosedimented with the indicated ribosomes in the absence and presence of 4 μM SecB were quantified by densitometry of the Coomassie-stained gel and normalization to the signal from the band corresponding to ribosomal protein S1. Confidence intervals shown by error bars represent one standard deviation from the average of three independent experiments.