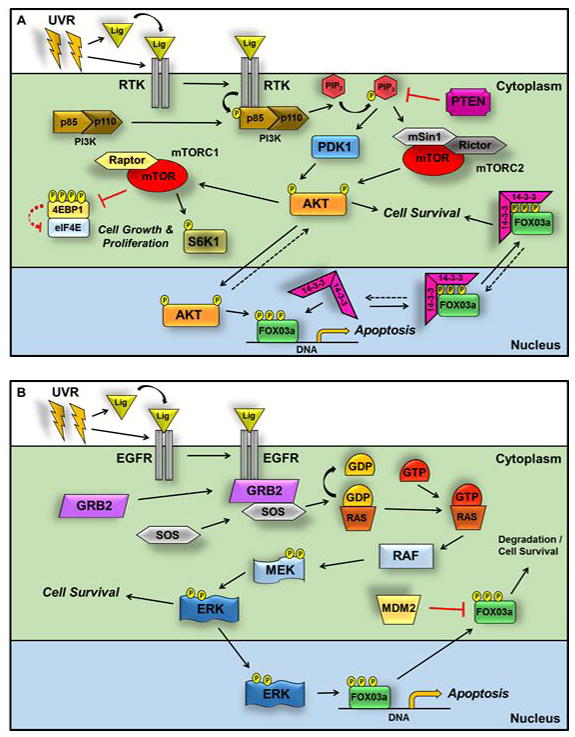
Figure 4. RTK activation by UVR results in an anti-apoptotic and cell proliferative response through PI3K/mTOR and Raf/MEK/ERK-dependent pathways.
(A.) Autophosphorylation of multiple UVR-inducible RTKs activates the PI3K heterodimer by recruitment to the receptor and binding of the p85 subunit SH2 domain. Phosphorylation of p85 at Tyr458 activates the heterodimer, and PI3K phosphorylates PIP2 to generate PIP3. PIP3 recruits PDK1 and AKT to the receptor complex, where PDK1 activates AKT by phosphorylation of Thr308. Once phosphorylated, AKT leads to mTORC1 activation (see text for details), which regulates proliferation and ribosome biogenesis by phosphorylation of S6K1 at Thr389 and cap-dependent translation by phosphorylation (at Thr37, Thr46, Ser65, and Thr70) and degradation of 4EBP1. PTEN negatively regulates PI3K-dependent signaling though PIP3 dephosphorylation and conversion to PIP2. To activate mTORC2, the PH domain of SIN1 binds to PIP3 to uncover the active kinase site of mTOR. PIP3 recruits AKT to mTORC2, and mTORC2 phosphorylates AKT at the hydrophobic motif site Ser473. Dual phosphorylation of AKT at Thr308 and Ser473 controls cell survival pathways by inhibition of pro-apoptotic proteins such as the FOXO3a transcription factor. AKT-dependent phosphorylation of FOXO3a at Thr32, Ser256 and Ser319 causes association of the 14-3-3ζ chaperone, which binds to this motif and sequesters FOXO3a in the cytoplasm. (B.) When Raf/MEK/ERK signaling is stimulated, GRB2 and SOS are recruited to the activated EGFR. Following exchange of GDP for GTP, the Ras GTPase induces RAF activation, which phosphorylates MEK1 and 2 at Ser217 and Ser221, respectively. ERK is activated by MEK-dependent phosphorylation at Thr202 and Tyr204 and regulates numerous downstream targets (see text for details), including FOXO3a. Phosphorylation of FOXO3a at Ser294, Ser344, and Ser425 triggers exit of FOXO3a into the cytoplasm in a 14-3-3-independent manner. In the absence of its association with 14-3-3, FOXO3a is targeted for MDM2-mediated cytoplasmic degradation.