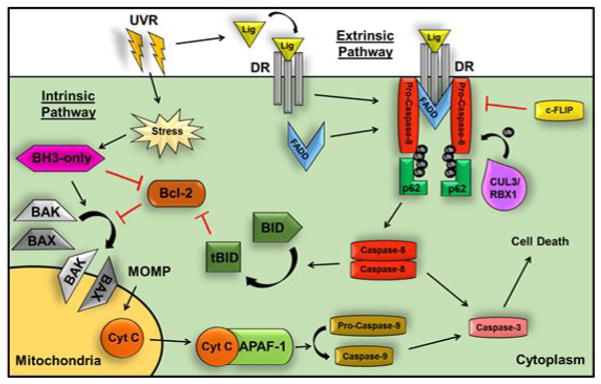
Figure 5. UVR can activate both intrinsic and extrinsic apoptosis.
Extrinsic apoptosis is initiated by ligand binding to DRs such as FasR and TRAIL-R1/2, which trimerize and recruit FADD to intracellular death domain binding sites. FADD then recruits the pro-caspase-8 dimer to form the DISC. Ubiquitination of pro-caspase-8 by CUL3/RBX1 allows binding of p62 and cleavage of pro-caspase-8 into its active form. The caspase-8 dimer causes the downstream activation of pro-caspase-3 to induce cell death. This process is inhibited by c-FLIP binding to FADD, which prevents caspase-8 dimerization and activation. Intrinsic apoptosis is activated by UVR in response to both DNA damage and hypoxia. In the absence of UVR, the activities of BAX and BAK are inhibited by Bcl-2 family proteins, whereas UVR exposure causes the BH3-only proteins to bind to and inhibit Bcl-2, allowing BAX and BAK to generate mitochondrial outer membrane permeabilization (MOMP) and subsequent release of cytochrome C into the cytosol. In the cytosol, cytochrome C binds APAF-1 to recruit and activate pro-caspase-9, which initiates caspase-3-dependent cell death. Caspase-8 can also cleave BID to generate tBID, which promotes mitochondrial-mediated cell death, thus linking the intrinsic and extrinsic pathways.