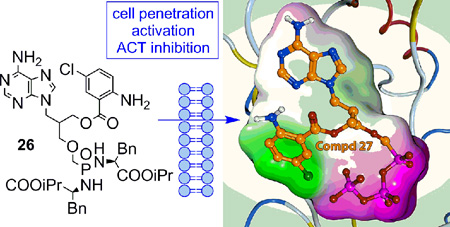
Abstract
Bordetella pertussis adenylate cyclase toxin (ACT) and Bacillus anthracis edema factor (EF) are key virulence factors with adenylate cyclase (AC) activity that substantially contribute to the pathogenesis of whooping cough and anthrax, respectively. There is an urgent need to develop potent and selective inhibitors of bacterial ACs with prospects for development of potential antibacterial therapeutics and to study their molecular interactions with the target enzymes. Novel fluorescent 5-chloroanthraniloyl-substituted acyclic nucleoside phosphonates (Cl-ANT-ANPs) were designed and synthesized in the form of their diphosphates (Cl-ANT-ANPpp) as competitive ACT and EF inhibitors with submicromolar potency (IC50 values 11 – 622 nM). Fluorescence experiments indicated that Cl-ANT-ANPpp analogues bind to the ACT active site and docking studies suggested that the Cl-ANT group interacts with Phe306 and Leu60. Interestingly, the increase of direct fluorescence with Cl-ANT-ANPpp having an ester linker was strictly CaM-dependent, whereas Cl-ANT-ANPpp analogues with an amide linker, upon binding to ACT, increased the fluorescence even in the absence of CaM. Such a dependence of binding on structural modification could be exploited in the future design of potent inhibitors of bacterial ACs. Furthermore, one Cl-ANT-ANP in the form of a bisamidate prodrug was able to inhibit B. pertussis ACT activity in macrophage cells with IC50 = 12 µM.
Graphical Abstract
Introduction
Whooping cough, or pertussis, is caused by a strictly human pathogen, Bordetella pertussis which is transmitted from infected to susceptible individuals through droplets by coughing or sneezing. Pertussis cases and deaths are reported annually among children and adults despite high vaccination coverage among infants in many countries.[1] According to WHO estimates in 2008 about 16 million cases of pertussis occurred worldwide, 95% of which were in developing countries and about 195,000 children died from this disease.[2] The highest incidence and mortality rate is among infants and children less than 12 months old.[3]
Pertussis is generally treated with antibiotics.[4] Macrolide antibiotics have been effective and constitute the mainstay of such treatment. Antibiotic treatment is effective in the first catarrhal stage, which is manifested by signs and symptoms resembling various viral infections and a pertussis diagnosis is frequently overlooked. However, antibiotic treatment in the later paroxysmal and convalescence stages is still recommended to clear the nasopharynx of B. pertussis and to prevent further spread of the infection. Antimicrobial resistance to B. pertussis has been reported sporadically.[3,5] Immunity to pertussis, acquired from natural infection or through vaccination, is not lifelong. Pertussis infection yields 3 to 30 years of protection and the estimated protection from the pertussis vaccine is 4 to 14 years depending on the vaccine. The whole-cell vaccine generally yields longer protection than the acellular vaccine. The severity of the disease is linked to the time since previous vaccination or illness.[3,6] Thus, the need for novel treatment strategies to treat pertussis still persists. One of the possible mechanisms to combat B. pertussis infections is the inhibition of one or more of the virulence factors of B. pertussis.
The calmodulin-dependent adenylate cyclase toxin (ACT) is considered an important virulence factor for B. pertussis.[7] This bifunctional hemolysin/adenylate cyclase protein consists of the N-terminal adenylate cyclase (AC) domain and the C-terminal RTX (Repeats in toxin cytolysin) hemolysin domain.[8] ACT binds to the surface receptors on phagocytes and translocates its AC domain into the cytosol. The endogenous Ca2+-sensor calmodulin (CaM) binds to ACT with high affinity which results in massive production of the cyclic AMP (cAMP) in host cells.[9] It causes nearly a 1,000-fold increase in the intracellular levels of cAMP and elevation of intracellular [Ca2+]i level.[10] The increase of cAMP inhibits phagocyte function and facilitates infection of respiratory tract by B. pertussis and other possible secondary pathogens.
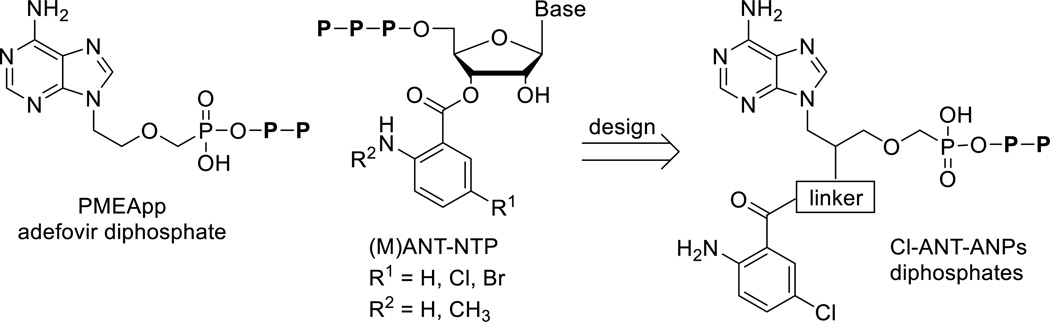
Acyclic nucleoside phosphonates (ANPs)[11] are established nucleotide analogues with a broad spectrum of biological activities. The most pronounced is their antiviral effect,[12] however ANPs exhibit also cytostatic,[13] antibacterial,[14] antiparasitic,[15] and immunomodulatory activities.[16] Adefovir diphosphate (PMEApp, Figure 1), the active cellular metabolite of adefovir dipivoxil (bis(POM)PMEA) approved for the treatment of chronic hepatitis B virus infection, inhibits Bordetella pertussis ACT[17] and Bacillus anthracis edema factor (EF),[18] both having adenylate cyclase activity. PMEA and its triphosphate derivatives were also described to inhibit rat brain adenylate cyclase activity.[19] Furthermore, 2-substituted PMEA derivatives proved to inhibit B. pertussis ACT, with promising selectivity for ACT over mammalian ACs and no cytotoxicity.[20] It was also shown that cell-permeable bisamidate prodrugs of PMEA bearing bis(l-phenylalanine isopropyl ester) moiety and its 2-substituted derivatives inhibited ACT effectively in cellular models. Although these bisamidate prodrugs did not inhibit ACT in vitro as effectively as bis(POM)PMEA, they were significantly less cytotoxic and showed better plasma stability profiles.[21]
Figure 1.
Structures of adefovir diphosphate (PMEApp), (M)ANT-nucleotides and target Cl-ANT-ANPpp analogues.
Gille and Seifert discovered (N-methyl)anthraniloyl-substituted nucleoside triphosphates, (M)ANT-NTPs (Figure 1) as potent competitive inhibitors of mammalian and bacterial ACs, including ACT.[22] Bis-Cl- and bis-Br-ANT-ATP and their ITP analogues were the most potent derivatives known, displaying 50- to 150-fold better selectivity for ACT over mACs.[22]
Based on the crystal structure of ACT with PMEApp (1ZOT),[17] we have proposed structures of new acyclic nucleoside phosphonates (ANPs) bearing 5-Cl-ANT substituent at 2’ position of the aliphatic side chain attached via variable linkers (Figure 1). Compared to (M)ANT-NTPs, which are suitable for studies of binding interactions with enzymes but are not therapeutically applicable due to their rapid degradation in vivo, ANPs are chemically and enzymatically stable phosphate analogues, which can be phosphorylated in vivo to their diphosphate forms. Thus, ANPs bearing anthraniloyl moiety may benefit both from their increased biological stability (compared to the natural nucleotides) and from their improved binding affinity to the enzyme (compared to standard ANPs) via additional interaction(s) of the aromatic moiety with the lipophilic pocket in the enzyme active site.
In the present study, we describe synthesis of racemic acyclic nucleoside phosphonates bearing 5-chloro-anthraniloyl substituent attached through various linkers (-O-, -NH-, -OCH2-, -NCH2-) to the acyclic chain of ANPs. Acyclic nucleoside phosphonate diphosphates (ANPpp), as nucleoside triphosphate analogues, were prepared for their evaluation in the enzyme assays and for fluorescence experiments. On the other hand, for in vitro and/or in vivo screening of biological properties, the polar phosphonate moiety of ANPs had to be masked by lipophilic prodrugs to facilitate their transport across cell membranes and, thus, improve their potential bioavalability. To this end, bis-amidate derivative bearing bis(l-phenylalanine isopropyl ester) moiety was selected as a prodrug for its advantageous stability and toxicity profile.[20,21]
Since (M)ANT-nucleosides triphosphates are environmentally sensitive probes displaying increased fluorescence and blue shift of the emission maximum upon exposure to a hydrophobic environment,[23] such analogues can be studied upon binding to the enzyme active site by measuring direct fluorescence and/or fluorescence resonance energy transfer (FRET). From the crystal structure of ACT with PMEApp,[17] it can be observed that the catalytic site contains hydrophobic residue Phe306. Binding of the (M)ANT-nucleotide analogues to ACT allows hydrophobic interactions between the (M)ANT group and Phe306, resulting in an increased fluorescence signal. Furthermore, the catalytic domain of ACT bears two tryptophan residues, Trp69 and Trp242, located near the catalytic site, which allow the inhibitor-enzyme complex to be studied using FRET.[24] Both fluorescence experiments and in silico studies of (M)ANT nucleotides showed that they bind to the active site of the enzyme and that hydrophobic interactions significantly contribute to their binding.[24] Some of the (M)ANT nucleosides were shown to bind to the ACT even in the absence of CaM.[24]
Results and discussion
Chemistry
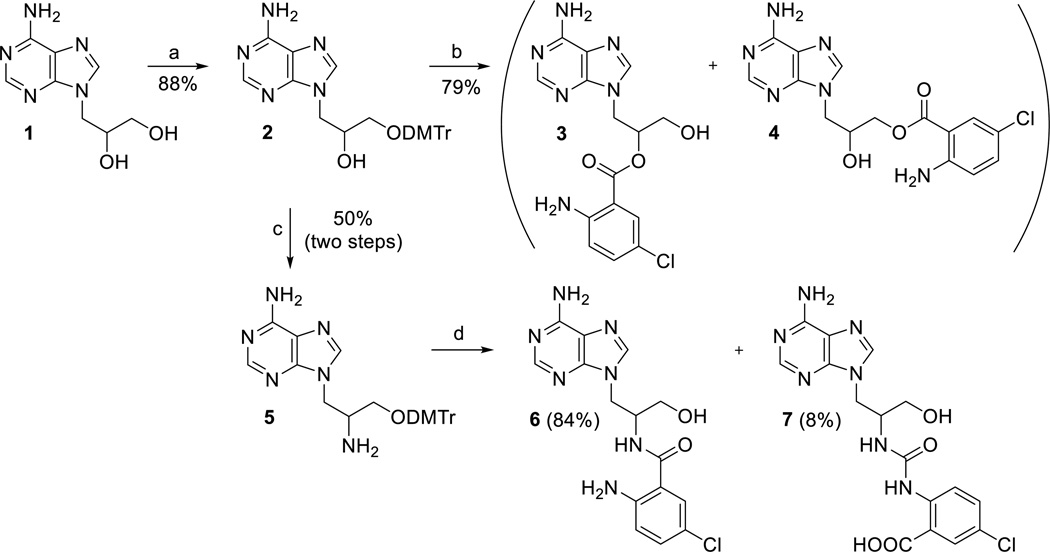
Synthesis of the target compounds started from 2’,3’-dihydroxypropyladenine[25] (1, Scheme 1) by protection of the primary 3’-OH group with DMTrCl in pyridine and subsequent reaction with 5-chloroisatoic anhydride in the presence of base.[26] However, upon deprotection of the DMTr group with acetic acid, the anthraniloyl moiety partially migrates from 2’-position to 3’-position to give an inseparable mixture of compounds 3 and 4 (Scheme 1) in a ratio 5:1 and in overall 79% yield. This isomerization was observed with MANT-nucleotides as well.[22] To prepare anthraniloyl derivative with more stable amidic bond, 2’-amino derivative 5 was prepared in overall 42% yield (3 steps) by conversion of the 2’-OH group to mesylate, azide, and finally to amino group (Scheme 1).[27] Treatment of compound 5 with 5-chloroisatoic anhydride under the above conditions and subsequent removal of the DMTr group afforded the desired compound 6 in only 12% yield, together with derivative 7 (75%, data not shown), which was formed by the ring opening of 5-chloroisatoic anhydride via an attack of amino derivative 5 at the carbamic acid carbonyl. Similar result was obtained when using TEA instead of NaH as a base. Finally, when compound 5 was treated with 5-chloroisatoic anhydride without any base, the desired derivative 6 was obtained as a major product (84% yield) with only traces (8%) of derivative 7 (Scheme 1).[28] Compared to ester compound 3, the amide analogue 6 was stable and did not undergo any isomerization.
Scheme 1.
Synthesis of the Cl-ANT acyclic nucleosides. Reagents and conditions: a) DMTrCl, Py, RT, overnight; b) i) NaH, DMF, 5-chloroisatoic anhydride, 14 h; ii) 80% AcOH, RT, 2h; c) i) MsCl, Py, RT, 2 h; ii) NaN3, DMF, HMPA, 100 °C, 14 h; iii) H2/Pd/C, MeOH, RT, 24 h; d) i) 5-chloroisatoic anhydride, DMF, THF; ii) 80% AcOH, RT, 2h.
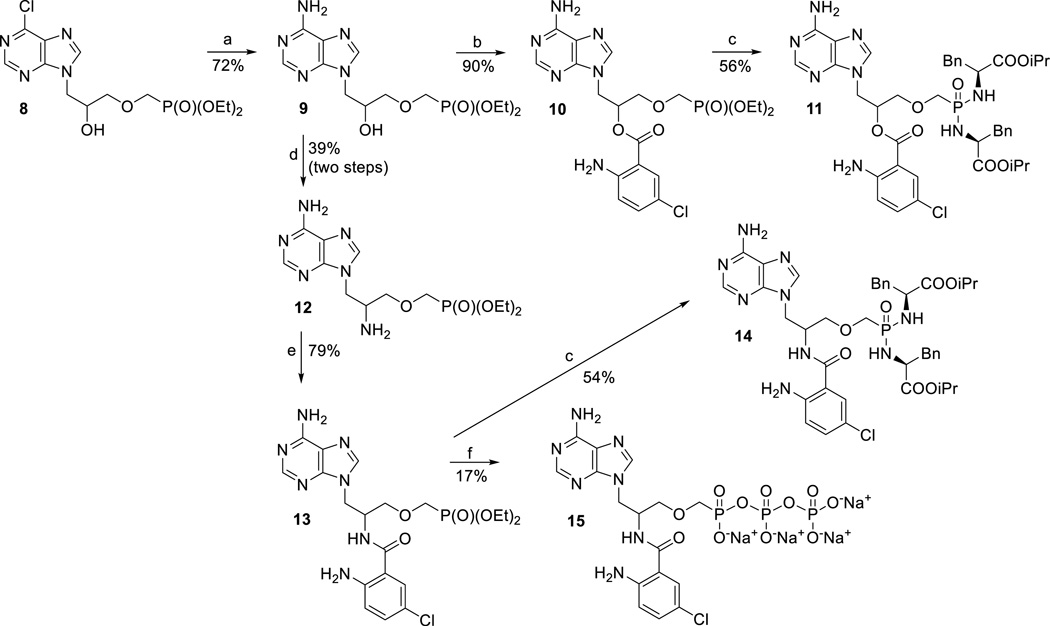
As all attempts to alkylate the 3’-OH group of compounds 3 or 6 with tosyl- or halomethylphosphonate were unsuccessful (data not shown), we decided to introduce the phosphonate moiety first, followed by an attachment of the Cl-ANT group later, as depicted in Scheme 2. The synthesis started by a conversion of previously reported 6-chloro derivative 8[29] to adenine analogue 9 using ammonia in ethanol.[30] 2’-Amino derivative 12 was prepared from compound 9 by the same procedure as compound 5 from 2 (Scheme 1). The subsequent reaction of compounds 9 and 12 with 5-chloroisatoic anhydride gave products 10 and 13, respectively (Scheme 2). Finally, phosphonates 10 and 13 were converted to the corresponding bis-amidates 11 and 14, respectively, by transsilylation and subsequent treatment with l-phenylalanine isopropyl ester under standard reaction conditions.[31] For enzymatic assays triphosphate analogue 15 was prepared from 13 employing the standard morpholidate methodology.[32] We also attempted to prepare the corresponding triphosphate analogue derived from compound 10, however the desired product proved to be unstable, probably due to formation of more stable cyclic phosphonate,[29] affording after work up a complex mixture.
Scheme 2.
Synthesis of Cl-ANT-ANPs. Reagents and conditions: a) NH3/EtOH, MW, 100 °C, 30 min; b) 5-chloroisatoic anhydride, NaH, THF, RT, 24 h; c) TMSBr, Py, RT, 12 h, then L-phenylalanine isopropyl ester hydrochloride, TEA, 2,2’-dipyridyldisufide, PPh3, Py, 70 °C, 72 h; d) i) MsCl, Py, RT, 2 h; ii) NaN3, DMF, HMPA, RT, 5 days; iii) H2/Pd/C, MeOH, RT, 12 h; e) 5-chloroisatoic anhydride, DMF, THF, DMAP, RT, overnight; f) i) TMSBr, Py, RT, 14 h; ii) morpholine, t-BuOH, DCC, H2O; III) tributylammonium pyrophosphate, DMSO, RT, 48 h.
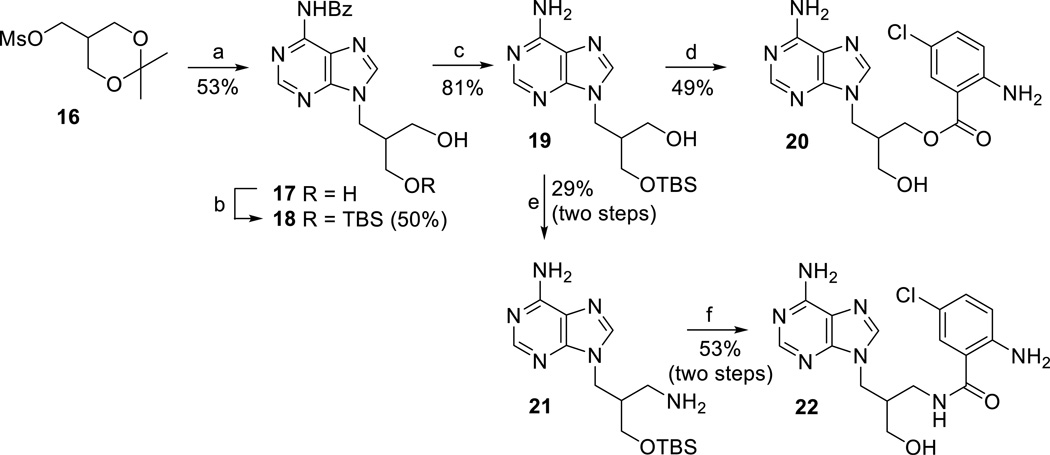
Target derivatives with a prolonged linker at 2’-position containing either –CH2O– or –CH2NH– linker were prepared in analogy to the above reported series of compounds, starting from racemic compound 16 (Scheme 3).[33] Treatment of N6-benzoyl adenine with compound 16 and NaH in DMF, followed by the removal of isopropylidene moiety with acetic acid (to give 17) and protection of one of the free hydroxyl groups using TBSCl[34] afforded derivative 18 (Scheme 3). Debenzoylation of compound 18 gave intermediate 19, which was further converted into 3’-amino derivative 21 by standard reaction sequence as described above. Then, the Cl-ANT group was introduced into derivatives 19 and 21, followed by the removal of the TBS groups, to give compounds 20 and 22, respectively (Scheme 3).
Scheme 3.
Synthesis of Cl-ANT acyclic nucleosides with extended linker. Reagents and conditions: a) i) N6-benzoyladenine, NaH, DMF, 60 °C, 14 h; ii) 80% AcOH, 60 °C, 30 min; b) TBSCl, imidazole, DMF, RT, 14 h; c) 1M NaOMe/MeOH, MeOH, RT, 16 h; d) i) 5-chloroisatoic anhydride, NaH, DMF, 80 °C, 3 h; ii) 80% AcOH, 50 °C, 8 h; e) i) MsCl, Py, RT, 2 h; ii) NaN3, DMF, RT, 5 days; iii) H2/Pd/C, MeOH, RT, 14 h; f) i) 5-chloroisatoic anhydride, DMF, THF, DMAP, RT, 16 h; ii) 80% AcOH, 50 °C, 2 h.
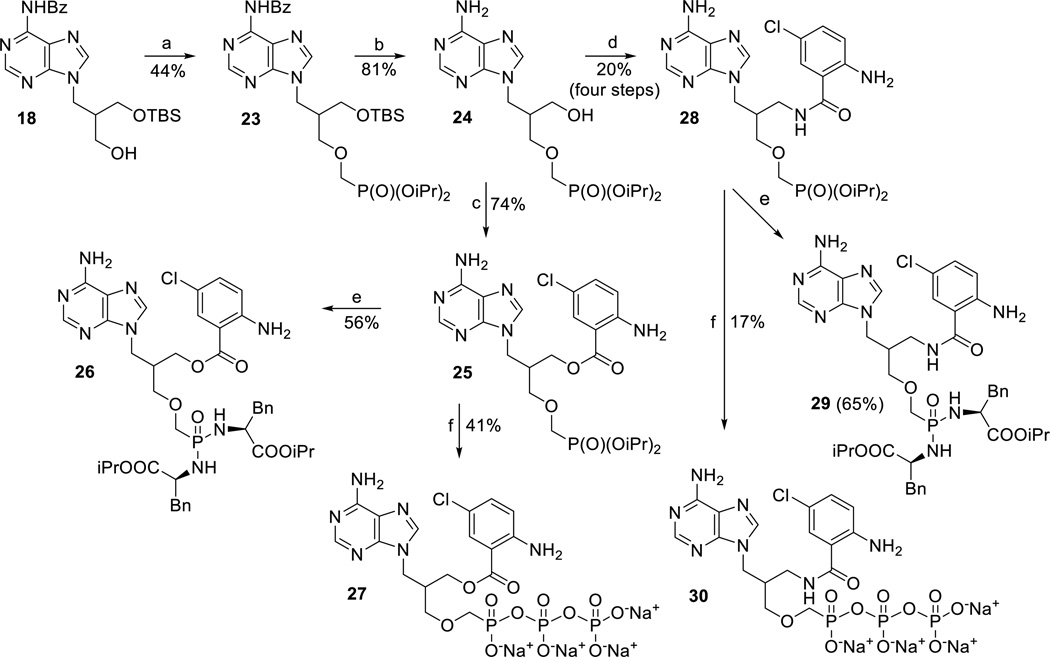
Finally, TBS-protected derivative 18 was alkylated at the 3’-OH group with bis(2-propyl) p-toluenesulfonyloxymethylphosphonate to give compound 23 (Scheme 4). After the TBS group removal, the hydroxyderivative 24 was converted to Cl-ANT derivatives 25 and 28 (Scheme 4) by above described procedures. For biological screening, phosphonates 25 and 28 were converted to their bis-amidates 26 and 29, respectively, and to their diphosphate analogues 27 and 30 (Scheme 4), respectively.
Scheme 4.
Synthesis of Cl-ANT-ANPs derivatives with extended linker. Reagents and conditions: a) pTsOCH2P(O)(OiPr)2, Mg(tBuO)2, DMF, 60°C, 72 h; b) TBAF, THF, RT, 16 h; c) 5-chloroisatoic anhydride, NaH, DMF, 50 °C, 48 h; d) i) MsCl, Py, RT, 2 h; ii) NaN3, DMF, RT, 5 days; iii) H2/Pd/C, MeOH, RT, 14 h; iv) 5-chloroisatoic anhydride, DMF, THF, DMAP, RT, 16 h; e) TMSBr, Py, RT, 12 h, then L-phenylalanine isopropyl ester hydrochloride, TEA, 2,2’-dipyridyldisufide, PPh3, Py, 70 °C, 72 h; f) i) TMSBr, Py, RT, 14 h; ii) morpholine, t-BuOH, DCC, H2O; III) tributylammonium pyrophosphate, DMSO, RT, 48 h.
Biological evaluation
Inhibition of ACT activity in the cell-free assay
The prepared triphosphate analogues 15, 27 and 30 were tested, along with PMEApp as a standard, for their inhibitory activity towards several bacterial ACs (Table 1): two commercially available adenylate cyclase toxins (from Sigma and Enzo), expressed recombinantly in E. coli, and edema factor (EF) from Bacillus anthracis.
Table 1.
IC50 values of Cl-ANT-ANPpp for ACT and EF.
| Compound | IC50 [nM][a] ACT Sigma |
IC50 [nM][a] ACT Enzo |
IC50 [nM][a] EF |
|---|---|---|---|
| PMEApp | 16.0 ± 0.2 | 13.6 ± 4.7 | 11.5 ± 2.6 |
| 15 | 294 ± 5 | 263 ± 37 | 68.9 ± 18.2 |
| 27 | 77.0 ± 18.1 | 198 ± 24 | 622 ± 124 |
| 30 | 90.5 ± 1.1 | 47.0 ± 0.7 | 140 ± 15 |
Data are the mean of at least three independent experiments.
Compound 15 showed the same inhibitory activity towards both ACTs tested, but it was weaker inhibitor than compounds 27 and 30 (Table 1). Compounds 27 and 30 inhibited ACT from Sigma with similar IC50 (77 and 91 nM, respectively), but there was a difference in inhibition of ACT from Enzo (IC50 198 and 47 nM, respectively).
Although both ACTs did not differ significantly in enzymatic and inhibitory AC activities measured in the cell-free assays, only the toxin provided by Enzo was able to elicit a high level of cAMP in these assays. Recombinant ACT produced in E. coli has been referred to have the equivalent catalytic AC activity to ACT produced by B. pertussis. However, expression of ACT in E. coli results in reduced hemolytic activity due to differential posttranslational fatty acylation of the hemolysin domain.[35] Iwaki et al.[36] reported that hemolysin domain can stimulate translocation of AC domain across cell membrane. Thus, the potential difference between acylation patterns of these two toxins could explain their dissimilar ability to produce cAMP inside the cells. Furthermore, steric effects of hemolysin domain can affect the access of Cl-ANT-ANPpp to the active site and thus cause discrepancies in the IC50 values for these two toxins.
There is a 25% sequence identity between the core domains of EF and ACT.[17] Since PMEApp was also shown to inhibit EF effectively,[18] the effect of analogues 15, 27 and 30 on the catalytic activity of EF was also evaluated. In this case, compound 15 showed the best inhibitory activity towards EF (IC50 = 69 nM), followed by 27 (IC50 = 613 nM) and 30 (IC50 = 140 nM) (Table 1).
Inhibition of ACT in the cell-based assay
All other prepared Cl-ANT derivatives, either without the phosphonate moiety (compounds 3+4, 6, 7, 20, and 22) or with the phosphonate moiety in the form of bisamidate prodrugs (compounds 11, 14, 26, and 29), were tested for their ability to inhibit ACT activity in J774A.1 macrophage cells (Table 2). The bisamidate prodrugs were prepared as diastereoisomeric mixtures, which were not further separated for their biological evaluation. The putative mechanism of bisamidate cleavage has been reported previously.[21b] Murine macrophage cells J774A.1 were incubated with various concentrations of the tested compounds and subsequently exposed to B. pertussis ACT. The cells were lysed, and the amount of cAMP was determined.
Table 2.
ACT inhibition and cytotoxic effects of bisamidate prodrugs of 5-chloroanthraniloyl-substituted acyclic nucleoside phosphonates in J774A.1 cells.
| Compound | IC50[µM][a] | Viability [%][b] |
|---|---|---|
| 3 + 4 | > 15 | ND |
| 6 | > 15 | ND |
| 7 | > 15 | ND |
| 11 | > 15 | ND |
| 14 | > 15 | ND |
| 20 | > 15 | ND |
| 22 | > 15 | ND |
| 26 | 12.1 ± 1.3 | 110 |
| 29 | > 15 | ND |
Data are the mean ± SD of at least three independent experiments.
Data are the percent cell viability at a fixed prodrug concentration (10 µM) versus untreated control; ND: not determined.
Only compound 26, where the Cl-ANT moiety is attached at 2’-position via –CH2O– linker, showed inhibitory activity towards ACT with IC50 of 12 µM (Table 2). This activity is two orders of magnitude lower than that of the ACT inhibition by analogous bisamidate prodrug of PMEA or its 2-purine substituted derivatives.[20] Compound 26 exhibited no cytotoxic effect in J774A.1 cells at a concentration of 10 µM for 5h (Table 2).
Although all triphosphate analogues 15, 27, 30 inhibited enzyme activity in the cell-free assay efficiently (Table 1), only prodrug 26 (corresponding to 27) exhibited moderate inhibitory properties in the J774A.1 cellular model. There are several key preconditions for ANPs in their bisamidate prodrug form to be biologically active towards ACT in the cell-based assay: a) penetration of bisamidate prodrug across cellular membranes; b) efficient cleavage of the prodrug moieties to release free phosphonic acid (ANP); and finally c) subsequent double phosphorylation to ANPpp, the analogue of natural nucleoside triphosphate (in our case the analogue of ATP) which exerts the biological activity. Efficiency of all these steps can largely influence the observed inhibitory activity of Cl-ANT-ANPs inside cell.
Isopropyl ester bis(l-phenylalanine) prodrugs of Cl-ANT-ANPs were prepared, because this prodrug moiety displayed optimal stability profiles in both plasma and macrophage homogenate in our previous studies.[20] Bisamidate prodrugs have been proven to be hydrolyzed by lysosomal carboxypeptidase cathepsinA (CatA),[37] which is highly expressed in macrophages.[38] Definitely, the degree of hydrolysis of compounds 11, 14, 26 and 29 by this enzyme may differ, as well as the extent of their phosphorylation by kinases.
Fluorescence experiments with Cl-ANT-ANPpp analogues
FRET Experiments with Cl-ANT-ANPpp analogues
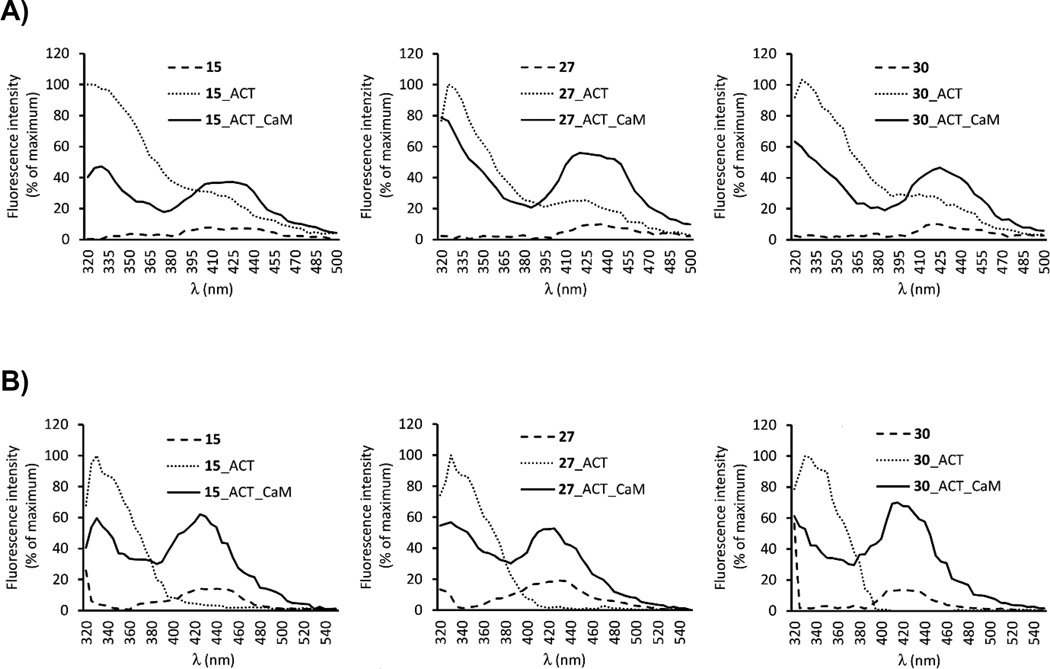
In FRET experiments, compounds 15, 27 and 30 were added first to the buffer, and their autofluorescence as a result of excitation at 280 nm was detected at 430 nm (Figure 2A, dashed lines). When ACT was added, tryptophan and tyrosine fluorescence occurred at 350 nm (Figure 2A, dotted line). Upon addition of CaM, FRET (decrease in emission at 350 nm and increase in emission at 430 nm) occurred (Figure 2A, solid lines). Similar results were observed when FRET experiments were performed using the tryptophan-specific excitation wavelength of 295 nm (Figure 2B).
Figure 2.
Emission spectra representing binding of 15, 27 and 30 to the catalytic site of ACT and the effect of calmodulin (CaM) on this process. Tested compounds were added to the assay buffer at final concentrations of 100 nM (27, 30) or 300 nM (15) and the emission was scanned following the excitation at 280 nm (A) and 295 nm (B) – dashed lines. ACT (dotted lines) or ACT and CaM (solid lines) were added successively to yield final concentration of 300 nM. Data are expressed as a percent of maximal fluorescence. Superimposed recordings of representative experiments are shown.
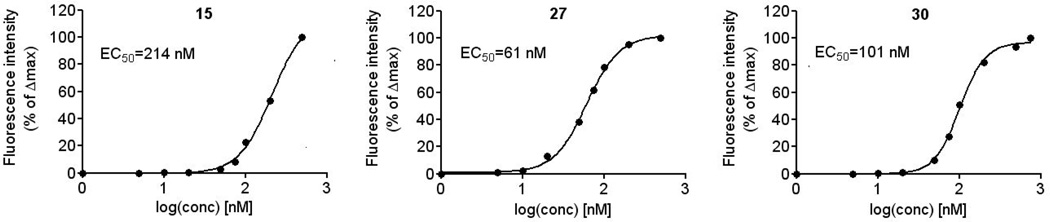
By determining FRET with Cl-ANT-ANPpp analogues at increasing concentrations after addition of CaM, saturation curves were obtained (Figure 3). Calculated EC50 values were 214 nM for 15, 61 nM for 27 and 101 nM for 30, which is in good agreement with the IC50 values for ACT inhibition obtained in the cell-free assay (Table 1).
Figure 3.
Saturation of ACT active site with 15, 27 and 30. Final concentrations of ACT and CaM were 300 nM of each, nucleotides were used at final concentrations from 10 nM to 500 nM. The fluorescence increase at 430 nm was calculated by subtracting the fluorescence at 430 nm after the addition of ACT to Cl-ANT-ANPpp analogues from the maximal fluorescence at 430 nm following the final addition of CaM to the mixture. Similar data were obtained in three independent experiments. Half-saturation concentration EC50 was calculated using GraphPad Prism 5.0 software.
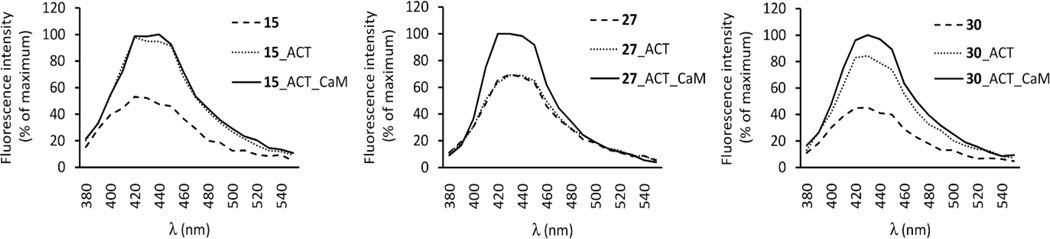
Direct fluorescence measurements
Tested compounds were excited at 350 nm and emission was scanned from 380 to 550 nm. Addition of ACT to the well containing 27 did not result in increase of its intrinsic fluorescence (Figure 4). Upon the addition of CaM, fluorescence immediately increased by 30% and the emission maximum of 27 showed a shift to shorter wavelengths (Figure 4). There was no change in fluorescence profile during the time (Figure S1). After the addition of ACT to the wells containing 15 and 30, their fluorescence immediately increased two times and did not change with time (Figure 4, Figure S1). Subsequent addition of CaM led to progressive increase in fluorescence of 15 and 30 by 16 times and 6 times, respectively, within 2 min (Figure 4, Figure S1). These results show that the Cl-ANT moiety apparently interacts with the hydrophobic moiety of Phe306 in the active site of ACT. CaM is essential for the binding of compound 27 to the ACT active site since 27 does not increase fluorescence in the absence of CaM. On the other hand, derivatives 15 and 30 increased fluorescence in the absence of CaM, which shows that these derivatives can bind to the active site even without CaM. However, after addition of CaM, fluorescence dramatically increased suggesting even more effective binding to the enzyme in the presence of CaM.
Figure 4.
Direct fluorescence of 15, 27 and 30. 100 nM of compound, 2.4 µM ACT, and 2.4 µM CaM were added to well in sequence. The excitation wavelength was 350 nm, and emission was immediately scanned from 380 to 550 nm. Superimposed recordings of a representative experiment are shown. Similar data were obtained in three independent experiments.
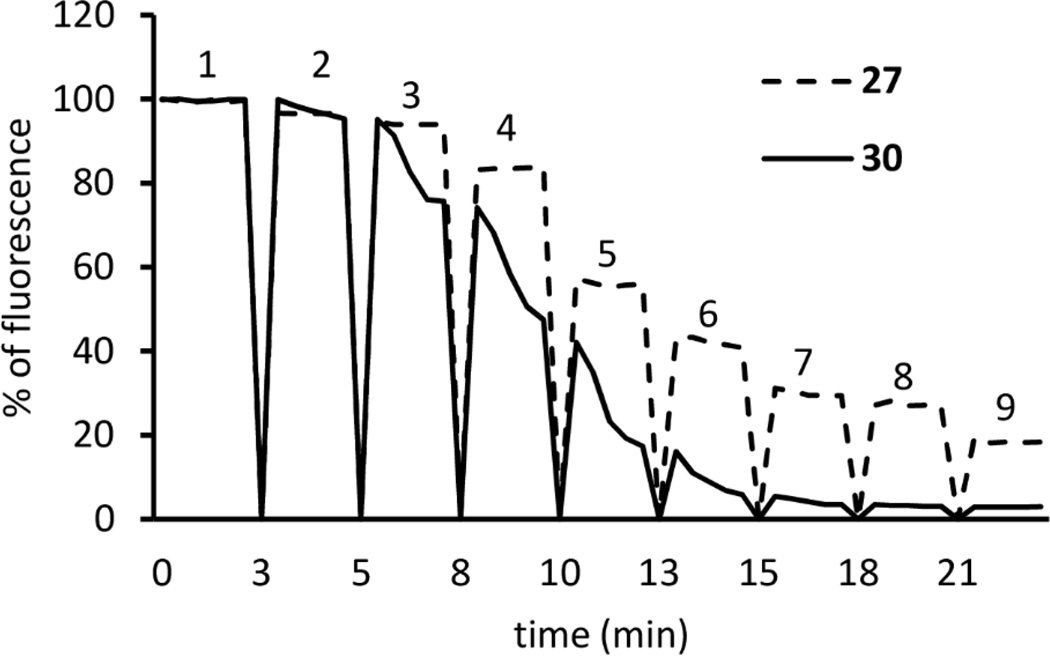
Inhibition of FRET by PMEApp
PMEApp inhibited FRET in a concentration-dependent manner (Figure 5). Half-maximal displacement of 100 nM 27 and 30 occurred at a PMEApp concentration of 67.05 ± 8.2 nM and 33.26 ± 5.9 nM, respectively. Kinetics of ACT FRET inhibition by PMEApp occurred within the mixing time in the case of 27. Displacement of 30 by PMEA was slower than that observed for 27.
Figure 5.
Time-resolved activation of ACT by CaM and stepwise abolishment of FRET by PMEApp. Excitation wavelength was 280 nm and emission was detected at 430 nm over time. 100 nM of 27 or 30, 300 nM ACT, 300 nM CaM, and PMEApp in the final concentrations of 0 nM (peak 1), 5 nM (peak 2), 25 nM (peak 3), 50 nM (peak 4), 100 nM (peak 5), 200 nM (peak 6), 300 nM (peak 7), 500 nM (peak 8) and 1000 nM (peak 9), were added in sequence. A record of a representative experiment is shown. Similar data were obtained in three independent experiments.
In conclusion, all compounds 15, 27 and 30 bind to the active site of ACT, as demonstrated by their displacement with PMEApp. Compounds 15 and 30 can bind to ACT in the absence of CaM, although CaM clearly stimulates their binding by an order of magnitude. Compounds 15 and 30 showed similar spectral changes that have been observed for 2,4,6-trinitrophenyl (TNP) nucleotides.[24] Compound 27 does not bind to ACT in the absence of CaM and it gives similar fluorescence response, and thus probably adopts similar position in the ACT binding site, as MANT nucleotide analogues.[24]
Molecular modeling of the binding mode of Cl-ANT-ANPpp to ACT
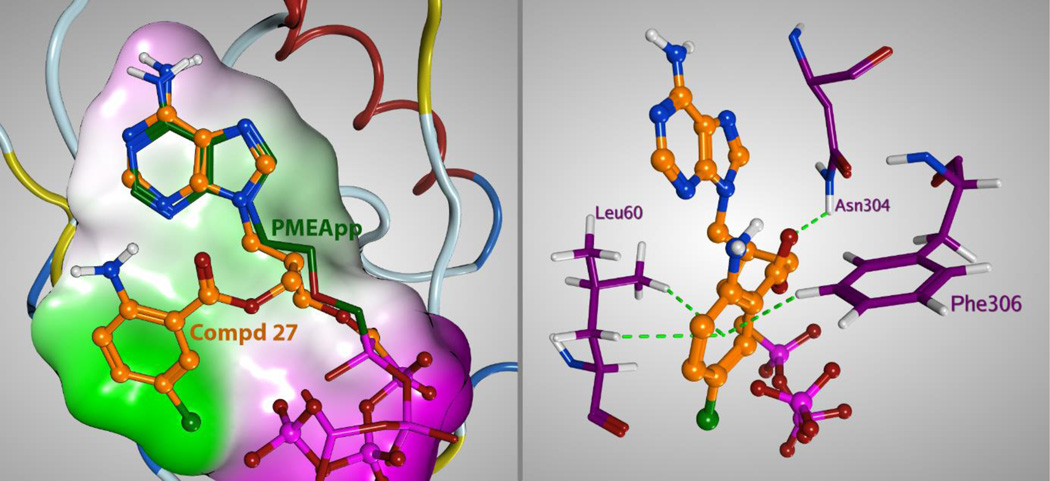
For further investigation of direct molecular space of ligands, docking studies were performed with Molecular Operating Environment (MOE).[39] The data obtained demonstrate (Figure 6) that the 5-chloroanthraniloyl (Cl-ANT) moiety interacts with Phe306 by C-H-π or possibly π-π interaction (in a distance 3.0Å to 3.1Å). Leu60 can also contribute to the binding of Cl-ANT moiety with C-H-π interaction (2.95–3.0Å). Only (S)-stereoisomers of compounds 27 and 30 could be placed into the binding pocket which indicates that only the (S)-stereoisomers are binding to the ACT active site and, thus, exert biological effects observed. Unfortunately, attempts to separate the individual stereoisomers of 27, using capillary electrophoresis with cyclodextrins as chiral selectors,[40] did not afford enough material for the evaluation of their discrete ACT inhibitory properties.
Figure 6.
Left: Comparison of docking pose of compound 27 with PMEApp in the crystal structure (PDB 1ZOT).[17] Right: Detail of binding of compound 27 in the ACT lipophilic pocket.
Another key interaction was observed between Asn304 residue and ester or amide linker of the Cl-ANT moiety (average distance of 2.6Å) in compounds 27 (Figure 6) and 30. The hydrogen bond between amide group of Asn304 and carbonyl of the ester linker apparently promotes strong binding of derivative 27 (Figure 6). Better inhibitory properties of amidic derivatives 15 and 30 in comparison with ester analogue 27 (Table 1), as well as slower displacement of 30 with PMEApp from the active site, can be speculated to be due to the presence of two possible intermolecular amide-amide (C=O…H-N) hydrogen bonds between compounds 15 or 30 and ACT.
Inhibition of mACs
Finally, the ability of the prepared compounds (3+4, 6, 7, 11, 14, 20, 22, 26 and 29) to inhibit host mammalian adenylate cyclases (mACs) was examined (Table 3). The assays were carried out using HEK293 cells stably expressing mAC1, mAC2, or mAC5, and each compound was tested in two independent experiments at 30 µM. These mACs are representatives of the three major families of mACs. Specific activation of the mAC overexpressed in the cells was accomplished by previously reported methodology,[41] where a calcium ionophore A23187 was employed to stimulate AC1, a PKC activator, phorbol 12-myristate 13-acetate (PMA) to stimulate AC2, and forskolin to stimulate AC5. None of the compounds significantly inhibited any mAC but several compounds (11, 14 and 29) slightly potentiated the selectively-stimulated cAMP response at AC1 and AC2 (Table 3).
Table 3.
Mammalian AC1, AC2 and AC5 inhibition with with prepared Cl-ANT-compounds at concentration of 30 µM). SQ22536 is a non-selective P-site inhibitor and SKF83566 is a selective inhibitors of AC2.[41]
| Compound | % of controla | ||
|---|---|---|---|
| AC1 | AC2 | AC5 | |
| 3+4 | 180±21 | 147±15 | 97±8 |
| 6 | 163±34 | 161±16 | 98±14 |
| 7 | 178±31 | 164±23 | 110±16 |
| 11 | 168±12 | 159±30 | 103±10 |
| 14 | 118±12 | 172±34 | 121±23 |
| 20 | 122±15 | 146±10 | 114±18 |
| 22 | 156±23 | 149±26 | 101±5 |
| 26 | 94±10 | 149±27 | 97±11 |
| 29 | 189±15 | 193±48 | 118±18 |
| SKF83566 | 95±9 | −2±4 | 83±10 |
| SQ22536 | 102±25 | 84±5 | 64±1 |
The AC-inhibition data are expressed as % ± S.E.M. of the control response (100%) in two independent experiments.
Conclusions
A novel series of potent inhibitors of bacterial adenylate cyclases (ACs), namely adenylate cyclase toxin (ACT) from B. pertussis and edema factor (EF) from B. anthracis, was designed. Three acyclic nucleoside phosphonate diphosphates with the Cl-ANT substituent attached to the acyclic side chain (Cl-ANT-ANPpp analogues), compounds 15, 27 and 30, were successfully prepared, while the corresponding ANPpp derived from compound 10 could not be isolated due to its instability. All three nucleoside triphophate analogues 15, 27 and 30 exhibited submicromolar inhibitory activity towards both ACT and EF with their potency comparable to that of (bis)-Cl-ANT-NTPs reported by the group of Seifert.[22] However, adefovir diphosphate (PMEApp) and its 2-substituted analogues still exhibit better inhibitory activities in the low nanomolar range.[20] The attachment of fluorescent Cl-ANT group to potential ACs inhibitors enables an examination of their binding using fluorescence methodology.[24] The fluorescence experiments with compounds 15, 27 and 30 showed similar binding to ACT as parent (M)ANT-nucleotides, which indicates their binding to the catalytic site of ACT. Furthermore, molecular docking experiments showed direct interactions of Cl-ANT-group with Phe306 and Leu60 in the active site, while the carbonyl group (C=O) present in the aliphatic linker of the ANPpp inhibitors is able to form an extra hydrogen bond with Asn304.
The ACT catalytic site is formed by a cavity with a substantial freedom to accommodate structurally diverse ligands, for example various purine, as well as pyrimidine nucleotide analogues.[24] On the other hand, even small structural variation can lead to a surprisingly huge difference in the binding mode of the ACT inhibitors. We have shown that compound 27 requires the presence of CaM to bind to ACT, while the other two potent ACT inhibitors, compounds 15 and 30, bind to ACT even in the absence of CaM, although CaM clearly stimulates their binding by an order of magnitude. This is an important observation. The results indicate slightly different orientations of the two types of studied Cl-ANT-ANPpp analogues (with ester or amide bond in the aliphatic chain) in the ACT catalytic site. Both types of compounds may provide valuable conformational probes to better understand the mechanism of ACT activation and, at the same time, can potentially facilitate the design of future potent and selective ACs inhibitors.
Finally, the corresponding Cl-ANT-ANPs 11, 14, 26 and 29, as isopropyl ester bis(l-phenylalanine) prodrugs,[20,21] were efficiently synthesized for their evaluation in cell-based assays. ACT inhibitory properties of these Cl-ANT-ANPs, as well as their acyclic nucleoside intermediates 3+4, 6, 7, 20 and 22, were studied in vitro in J774A.1 macrophages. From the compounds tested, only prodrug 26, corresponding to the potent triphosphate analogue 27, exhibited reasonable inhibitory activity towards ACT with an IC50 value of 12 µM and no observed cytotoxic effects in J774A.1 cells. The lack of potent inhibitory properties of the other prepared bisamidate prodrugs of ANPs, together with no observed inhibition of mammalian ACs (mAC1, mAC2 and mAC5) in cell-based assays, may suggest that the released ANPs are not sufficiently phosphorylated in the cells to the active species, i.e. ANPpp derivatives. This issue is going to be addressed in our future research.
In summary, Cl-ANT-ANPpp derivatives, acyclic analogues of fluorescent natural nucleoside triphosphates, represent a great tool to study molecular interactions of B. pertussis ACT with its substrates and/or potential inhibitors. The data obtained indicate that specific inhibitors can be designed that are able to interact with bacterial cyclases either in the presence or in the absence of CaM. Potent and selective inhibitors of bacterial adenylate cyclases with improved bioavailability may eventually become valuable agents for prophylaxis or reduction of toxemia in highly contagious diseases like pertussis and anthrax.
Experimental Section
Starting compounds and other chemicals were purchase from commercial suppliers or prepared according to the published procedures. Solvents were dried by standard procedures. Solvents were evaporated at 40 °C/2 kPa. Analytical TLC was performed on plates of Kieselgel 60 F254 (Merck). NMR spectra were recorded on Bruker Avance 500 (1H at 500 MHz, 13C at 125.8 MHz) and Bruker Avance 400 (1H at 400 MHz, 13C at 100.6 MHz) spectrometers with TMS or dioxane (3.75 ppm for 1H, 67.19 ppm for 13C NMR) as internal standard or referenced to the residual solvent signal. Mass spectra were measured on UPLC-MS (Waters SQD-2). HR MS were taken on a LTQ Orbitrap XL spectrometer. The microwave-assisted reactions were carried out in CEM Discover (Explorer) microwave apparatus. Preparative HPLC purification of triphosphate analogues was performed on a column packed with POROS® HQ 50 mm (50mL) with use of a gradient of TEAB in water (0.05–0.5 M). The purity of the tested compounds was determined by HPLC (H2O-CH3CN, linear gradient) and was higher than 95%.
General procedures
General procedure 1 (GP1)
Reaction of a hydroxy derivative with 5-chloroisatoic anhydride: A hydroxy derivative (1 mmol) in dry DMF (8 mL) was treated with NaH (44 mg, 1.1 mmol) under Ar at RT and the resulting mixture was stirred at room temperature for 30 min. 5-Chloroisatoic anhydride (0.4 g, 2 mmol) was added and the mixture was stirred at 80 °C for 3 h. The reacting mixture was cooled to room temperature and poured to EtOAc (50 mL) and extracted twice with saturated NaHCO3 (50 mL) and brine (50 mL) and dried over MgSO4.
General procedure 2 (GP2)
Conversion of hydroxy group to amino group: The hydroxy derivative (1 mmol) was co-evaporated with dry pyridine (1 × 10 mL), dissolved in dry pyridine (10 mL) and treated with MsCl (0.15 mL, 2 mmol) at 0 °C and the reaction mixture was stirred at room temperature for 2 h. MeOH (5 mL) was added at 0 °C and the volatiles were evaporated. The crude product was dissolved in EtOAC (20 mL) and washed with NaHCO3 (20 mL), brine (20 mL) and dried over MgSO4. The obtained crude mesylate was without further purification dissolved in DMF/HMPA mixture (1:1, 6 mL) and treated with NaN3 (325 mg, 5 mmol) at 100 °C overnight, cooled and poured into EtOAc (50 mL), washed with brine (5 × 30 mL) and dried over MgSO4. The azido derivative was purified by flash chromatography (CHCl3/MeOH 0–5%). The azide (3.9 mmol) in MeOH (45 mL) was treated with H2 over Pd/C (10 wt. % loading, 350 mg) under atmospheric pressure for 24 h. The reaction mixture was filtered, evaporated and purified by flash chromatography (CHCl3/MeOH 0–5%).
General procedure 3 (GP3)
Reaction of amino derivative with 5-chloroisatoic anhydride: The amino derivative (0.3 mmol) in DMF/THF mixture (1:5, 3 mL) was treated with DMAP (3.7 mg, 0.03 mmol) and 5-chloroisatoic anhydride (0.12 g, 0.6 mmol) at room temperature overnight and solvents were evaporated.
General procedure 4 (GP4)
Preparation of the bisamidate prodrugs: Phosphonate diester (1 mmol) was dissolved in dry pyridine (10 mL), and TMSBr (1 mL) was added. The reaction mixture was stirred at room temperature overnight. After evaporation of the volatiles, the flask was purged with Ar (without any contact with air) and amino acid ester hydrochloride (4 mmol, dried in vacuo at 30 °C and 0.1 mbar for 1 day), dry trimethylamine (2 mL) and dry pyridine (8 mL) were added, and the mixture was heated at 70 °C to obtain a homogenous solution. Then, a solution of 2,2’-dipyridyldisulfide (6 mmol) and triphenylphosphine (6 mmol) in dry pyridine (10 mL) was added under Ar. The resulting mixture was heated at 70 °C for 72 h. After cooling to room temperature, the solvent was removed and the residue was purified by flash chromatography (gradient of MeOH (2–30%) in a mixture of hexane/EtOAc, 60:40) to remove impurities, followed by reversed-phase chromatography on C18 silica gel (aqueous MeOH 1–100%). The products were freeze dried from 1,4-dioxane.
General procedure 5 (GP5)
Synthesis of phosphonate diphosphates: Diisopropyl ester of phosphonic acid (0.1 mmol) in pyridine (2 mL) was treated with TMSBr (0.1 mL) at room temperature overnight. The volatiles were removed under reduced pressure, the residue was co-evaporated with water and suspended in water and the free phosphonic acid was filtered off, dissolved in tBuOH and H2O (1:1, 2 mL) and morpholine (35 µL) was added. Solution of DCC (82 mg) in tBuOH (2 mL) was added dropwise at 80 °C and the resulting mixture was heated at 80 °C until complete conversion of the reaction. The mixture was poured into H2O/Et2O mixture and the aqueous phase was washed with Et2O (3 × 30 mL), finally Et2O was washed with water. Collected aqueous phase was evaporated and the residue was co-evaporated with EtOH and dry toluene. The dried residue was treated with tri-n-butylammonium pyrophosphate (0.5 M solution in DMSO) at room tempertaure for 48 h. The reaction mixture was diluted with Et2O (10 mL), Et2O layer was poured off and the precipitate was dissolved in 0.05 M TEAB (4 mL) and applied onto a column of POROS® HQ, and eluted with a linear gradient of TEAB (0.05–0.5 M). The fractions containing product were concentrated at 27 °C and the residue was applied onto DOWEX 50 × 8 (Na+ form), eluted with water and freeze dried.
9-[3-O-(4,4’-dimethoxytrityl)-2-hydroxypropyl]adenine (2)
9-(2,3-dihydroxypropyl)adenine (4.18 g, 20 mmol) in dry pyridine (200 mL) was treated with DMAP (35 mg, 0.29 mmol), TEA (2.8 mL, 20 mmol) and DMTrCl (7.53 g, 22 mmol) at room temperature overnight. MeOH (10 mL) was added and the solvent was evaporated, the residue in EtOAc (100 mL) was washed with sat. NaHCO3 and brine and dried over MgSO4. The crude product was purified by flash chromatography (CHCl3 with 0.5% TEA/MeOH 0–5%) to give an off-white foam (6.1 g, 88%).
9-[2-O-(5-Chloroanthraniloyl)-3-hydroxypropyl)]adenine (3) and 9-[3-O-(5-chloroanthraniloyl)-2-hydroxypropyl)]adenine (4)
Prepared from 2 (708 mg, 1.38 mmol) according to GP1. The crude product was treated with AcOH (80%, 20 mL) at room temperature for 2h. Acetic acid was evaporated and the resulting crude product was purified by flash chromatography (CHCl3/MeOH 0–5%) and freeze dried from 1,4-dioxane to give inseparable mixture of compounds 3 and 4 (5:1, 396 mg, 79% overall yield).
9-[2-Amino-3-O-(4,4’-dimethoxytrityl)propyl]adenine (5)
Prepared from 2 by GP2. White foam, overall yield (two steps) 50%. Also, intermediate 9-[2’-azido-3’-O-(4,4’-dimethoxytrityl)propyl]adenine was isolated as a white foam, yield 85%.
9-[2-N-(5-Chloroanthraniloyl)-3-hydroxypropyl)]adenine (6) and 2-(3-(1-(6-amino-9H-purin-9-yl)-3-hydroxypropan-2-yl)ureido)-5-chlorobenzoic acid (7)
Prepared according to GP3. The crude product was treated with 80% AcOH (10 mL) at room temperature for 3 h. Acetic acid was evaporated and the residue was purified by flash chromatography (CHCl3/MeOH 0–5%) and freeze dried to give 6 (167 mg, 84%) as a white foam. As a second product compound 7 (10 mg, 8%) was isolated as a white foam.
9-{3-[(Diethoxyphosphoryl)methoxy]-2-hydroxypropyl}adenine (9)
Compound 8 (800 mg, 2.1 mmol) was treated with ammonia in EtOH (15 mL) and heated under microwave irradiation (50 W) at 100 °C for 1 h. The solvent was evaporated and the residue was purified by flash chromatography (CHCl3/MeOH 0–5%) to give 9 as a colorless oil (550 mg, 72%).
9-{3-[(Diethoxyphosphoryl)methoxy]-2-O-(5-chloroanthraniloyl)propyl}adenine (10)
Prepared from 9 (360 mg, 1 mmol) by GP1 at room temperature, purified by flash chromatography (CHCl3/MeOH 0–5%) to give yellowish oil (462 mg, 90%).
Bis(l-phenylalanine isopropyl ester) prodrug of ((3-(6-amino-9H-purin-9yl)-2-O-(5-chloroanthraniloyl)propoxy)methyl)phosphonic acid (11)
Prepared from 10 (110 mg, 0.2 mmol) by GP4, white foam (95 mg, 56%).
9-{-2-Amino-3-[(diethoxyphosphoryl)methoxy]propyl}adenine (12)
The hydroxy derivative 9 (550 mg, 1.5 mmol) in dry pyridine (10 mL) was treated with MsCl (0.22 mL, 3 mmol) at 0 °C a allowed to stir at room temperature for 2 h. MeOH (5 mL) was added at 0 °C and the volatiles were evaporated. The crude product was dissolved in EtOAC (20 mL) and washed with NaHCO3 (20 mL), brine (20 mL) and dried over MgSO4. The mesylate was without further purification dissolved in DMF/HMPA mixture (1:1, 5 mL) and treated with NaN3 (487 mg, 7.5 mmol) and stirred at room temperature for 3 days, further NaN3 (487 mg, 7.5 mmol) was added and the mixture was stirred for further 3 days and the resulting mixture was poured into EtOAc (50 mL) and washed with brine (5 × 30 mL) and dried over MgSO4 and purified by flash chromatography (CHCl3/MeOH 0–5%) to give 9-{-2-azido-3-[(diethoxyphosphoryl)methoxy]propyl}adenine (398 mg, 59%). The azide (190 mg, 0.49 mmol) in MeOH (10 mL) was treated with Pd/C (10 wt. % loading, 15 mg) and H2 under atmospheric pressure for 24 h. The reaction mixture was filtered, evaporated and purified by flash chromatography (CHCl3/MeOH 0–30%) to give colorless oil (120 mg, 66%).
9-{3-[(Diethoxyphosphoryl)methoxy]-2-N-(5-chloroanthraniloyl)propyl}adenine (13)
Prepared from 12 (115 mg, 0.32 mmol) by GP3 to give 13 as a colorless oil (130 mg, 79%).
Bis(l-phenylalanine isopropyl ester) prodrug of ((3-(6-amino-9H-purin-9yl)-2-N-(5-chloroanthraniloyl)propoxy)methyl)phosphonic acid (14)
Prepared from 13 (65 mg, 0.126 mmol) by GP4, white foam (58 mg, 54%).
((3-(6-Amino-9H-purin-9yl)-2-N-(5-chloroanthraniloyl)propoxy)methyl)phosphonic acid diphosphate, sodium salt (15)
Prepared from 13 (51.2 mg, 0.1 mmol) by GP5, white foam (12 mg, 17%).
9-(3-Hydroxy-2-(hydroxymethyl)propyl)-N6-benzoyladenine (17)
N6-benzoyladenine (1.84 g, 7.7 mmol) in dry DMF (25 mL) was treated with NaH (0.372 g, 9.3 mmol, 60% susp. In mineral oil) at 0 °C for 30 min. and compound 16 (2.6 g, 11.6 mmol) in dry DMF (5 mL) was added and the reaction mixture was heated at 60 °C for 24 h. The resulting mixture was cooled and diluted with EtOAc (200 mL) and washed with brine (3 × 10 mL) and dried over MgSO4. The crude product was treated with 80% AcOH (25 mL) at 60 °C for 30 min. The mixture was cooled and acetic acid was evaporated and co-evaporated with water and EtOH. Flash chromatography gave 17 (1.3 g, 53%) as a white solid.
9-(3-O-(t-Butyldimethylsilyl)-2-(hydroxymethyl)propyl)-N6-benzoyladenine (18)
Dihydroxy derivative 17 (1.48 g, 4.5 mmol) in dry DMF (25 mL) was treated with imidazole (0.46 g, 6.75 mmol) and TBSCl (0.75 g, 4.95 mmol) was added in portions and the resulting mixture was stirred at room temperature for 24 h. The solvent was evaporated and the crude product was purified by flash chromatography (CHCl3/MeOH 0–10%) to give 18 (1 g, 50%) as a white solid.
9-(3-O-(t-Butyldimethylsilyl)-2-(hydroxymethyl)propyl)adenine (19)
Compound 18 (830 mg, 1.9 mmol) in MeOH (15 mL) was treated with MeONa (2 mL, 1M solution in MeOH) at room temperature overnight. The reaction mixture was neutralized with AcOH and evaporated. The crude product was purified by flash chromatography (CHCl3/MeOH 0–10%) to give 19 (510 mg, 81%) as a white solid.
9-(3-O-(5-Chloroanthraniloyl)-2-(hydroxymethyl)propyl)adenine (20)
Prepared from 19 (170 mg, 0.5 mmol) by GP1. The crude product was treated with AcOH (80%, 10 mL) at 50 °C for 8 h. Acetic acid was evaporated, the residue was co-evaporated with water and EtOH and purified by flash chromatography (CHCl3/MeOH 0–10%) and freeze dried from 1,4-dioxane to give 20 (93 mg, 49%) as a white foam.
9-(2-(Aminomethyl)-3-O-(t-butyldimethylsilyl)propyl)adenine (21)
Prepared from 20 (120 mg, 0.36 mmol) by the same procedure as compound 12. Intermediate 9-(2-(azidomethyl)-3-O-(t-butyldimethylsilyl)propyl)adenine was obtained as a white solid (110 mg, 85%). The amino derivative (21) was obtained as a white solid (35 mg, 34%).
9-(3-N-(5-Chloroanthraniloyl)-2-(hydroxymethyl)propyl)adenine (22)
Prepared from 21 (35 mg, 0.1 mmol) by GP3. The crude product was treated with AcOH (80%, 5 mL) at 60 °C for 3 h. The solvent was evaporated, co-distilled with water and EtOH and the residue was purified by flash chromatography (CHCl3/MeOH 0–10%) and freeze dried from 1,4-dioxane to give 22 (27 mg, 53%) as a white foam.
9-(3-O-(t-Butyldimethylsilyl)-2-(diisopropoxyphosphorylmethoxymethyl)propyl)-N6-benzoyladenine (23)
Compound 18 (0.5 g, 1.13 mmol) in DMF (10 mL) was treated with (tBuO)2Mg (0.29 g, 1.7 mmol) and pTsOCH2P(O)(OiPr)2 (0.54 g, 1.7 mmol), and the reaction mixture was heated at 60 °C for 3 days. The mixture was cooled to room temperature, diluted with EtOAc (100 mL), washed with brine (3 × 10 mL) and dried over MgSO4. The product was purified by flash chromatography (CHCl3/MeOH 0–5%), colorless oil (380 mg, 44%).
9-[2-(Hydroxymethyl)-3-(diisopropoxyphosphorylmethoxy)propyl)]adenine (24)
Compound 23 (1.14 g, 1.8 mmol) in dry THF (50 mL) was treated with TBAF (1M solution in THF, 4 mL) at room temperature overnight, evaporated, dissolved in EtOAc and washed with brine and dried over MgSO4 to give a colorless oil: MS-ESI m/z (%): 506.16 (100) [M+H]+. The crude product was dissolved in MeOH (15 mL) and treated with MeONa (1M solution in MeOH, 3 mL) at room temperature for 6 h. The reaction mixture was neutralized with AcOH, evaporated and purified by flash chromatography (CHCl3/MeOH 0–5%). Compound 24 was obtained as a colorless oil (598 mg, 81%).
9-[2-(5-Chloroanthraniloyloxymethyl)-3-(diisopropoxyphosphorylmethoxy)propyl)]adenine (25)
Prepared from derivative 24 (400 mg, 1 mmol) by GP1, colorless oil (410 mg, 74%).
Bis(l-phenylalanine isopropyl ester) prodrug of ((3-(6-amino-9H-purin-9yl)-2-(5-chloroanthraniloyloxymethyl)propoxy)methyl)phosphonic acid (26)
Prepared from 25 (110 mg, 0.2 mmol) by GP4 to give 26 (95 mg, 56%) as a white foam.
((3-(6-Amino-9H-purin-9yl)-2-(5-chloroanthraniloyloxymethyl)propoxy)methyl)phosphonic acid diphosphate, sodium salt (27)
Prepared from 25 (55 mg, 0.1 mmol) by GP5, white foam, yield 29 mg (41%).
9-[2-(5-Chloroanthraniloylaminomethyl)-3-(diisopropoxyphosphorylmethoxy)propyl)]adenine (28)
The hydroxy derivative 24 (570 mg, 1.4 mmol) was converted to 9-[2-(azidomethyl)-3-(diisopropoxyphosphorylmethoxy)propyl)]adenine (280 mg, 46%) and further to 9-[2-(aminomethyl)-3-(diisopropoxyphosphorylmethoxy)propyl)]adenine (130 mg, 51%) by the same procedure as was described for compound 12. The amino derivative (120 mg, 0.3 mmol) was finally converted by GP3 to 28 (140 mg, 84%).
Bis(l-phenylalanine isopropyl ester) prodrug of ((3-(6-amino-9H-purin-9yl)-2-(5-chloroanthraniloylaminomethyl)propoxy)methyl)phosphonic acid (29)
Prepared from 28 (70 mg, 0.126 mmol) by GP4 to give 29 (70 mg, 65%) as a white foam.
((3-(6-Amino-9H-purin-9yl)-2-(5-chloroanthraniloylaminomethyl)propoxy)methyl)phosphonic acid diphosphate, sodium salt (30)
Prepared from 28 (55 mg, 0.1 mmol) by GP5, white foam, yield 12 mg (17%).
Molecular Docking
Crystal structure of Bordetella pertussis adenylyl cyclase toxin with CAM and PMEApp (PDB code 1ZOT, resolution 2.2Å)[17] was prepared with MOE Ligx with default setup and structure was minimized to RMS gradient of 0.001. Structures of all final compounds above were properly protonated and deprotonated and minimized to RMS gradient of 0.001. For docking studies rigid dock protocol was chosen with structure waters included and ligands rotate bonds was enabled. Default placement and refinement method was used with 50 retained structures after the first refinement and 20 retained structures after the second refinement. As positions of nucleobases in enzymes are typically highly conserved, pharmacophore restrains were applied for nucleobase (features volume in brackets): both aromatic rings (1.3), hydrogen donor to backbone oxygen of Thr300 (1.5), hydrogen acceptor from water1030 to nitrogen N1 of purine (1.3). For all calculations Amber12:EHT mixed forcefield was used with Generalized Born solvent model.
Biological assays
Effect on the viability of J774A.1 cells
J774A.1 cells were plated onto white 96-well assay plates at 5×104 cells per well and allowed to attach overnight. Cells were then washed with HBSS and treated with 10 µM compounds for 5 h. Cell viability was then assessed with a Cell Titer-Glo Luminescent Cell Viability assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Measurement of luminescence signal was performed by use of a GENios microplate reader (Tecan Systems). Data were expressed as percent of control, represented by untreated cells.
Inhibition of ACT – cell-based assay
J774A.1 cells were seeded in a 96-well plate at 5×104 cells per well and left to attach overnight. Prior to the experiment, cells were washed with HBSS (135 mM NaCl, 5.9 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 25 mM glucose, 10 mM HEPES [pH 7.4]) and pre-incubated with compounds at concentrations of 0.001–30 µM for 5 h. After that, cells were exposed to ACT (2 nM) from B.pertussis (Enzo Life Sciences, Palo Alto, CA; SA=115 µmol/min/mg) for 30 min. Finally, the cAMP content was determined by using the CatchPoint cAMP immunoassay kit (Molecular Devices, Wokingham, UK). After the addition of lysis buffer (50 µL per well) provided by the manufacturer, the cellular content was extracted by shaking the plate at 250 rpm for 10 min. The plate was then centrifuged to remove cell debris, the supernatant was replaced to the assay plate, and immunoassays were carried out according to the manufacturer’s instructions. Fluorescence signal was acquired using an Infinite M1000 plate reader (Tecan Systems Inc., San Jose, CA, USA).
Inhibition of ACT – cell-free assay
In a cell-free assay, AC enzymatic activity was measured by conversion of [3H] ATP to of [3H]cAMP. The reaction was carried out at 30°C for 30 min, with a final reaction volume of 50 µl. Each assay mixture contained 3 µM BSA, 20 mM HEPES (pH 7.4), 10 mM MnCl2, 1 mM EDTA, 1 µM CaCl2, 0.1 mM cold ATP, 20 µCi [2,8-3H]ATP (ARC, St. Louis, MO, USA; specific activity 20 Ci/mmol), 1.2 µM calmodulin and tested compound at concentration of 0 – 100 µM. Inhibition of AC activity was determined in the presence of 3 different enzymes ACT (Sigma, specific activity 65 µmol/min/mg), ACT (Enzo, specific activity 115 µmol/min/mg) and EF (LBL, specific activity 830 µmol/min/mg) with the final enzyme concentration of 1.1 nM , 0.67 nM and 0.12 nM , respectively. The incubation was carried out for 30 min at 30°C, in a final reaction volume of 50 µl. A 2 µL aliquot of the assay mixture was spotted on a polyethylenimine chromatographic sheet, and developed in 4M LiCl:1 M acetic acid (1:4). After developing, the spots containing ATP and cAMP were quantified using Radio-TLC scanner RITA (RAYTEST, Germany) with evaluation software GINA STAR TLC. Data were calculated from the percentage conversion of [3H]ATP to [3H]cAMP. Ki values were calculated using the Graphpad Prism 5 software (San Diego, CA, USA). All assays were performed in duplicate with three independent repetitions. In statistical analysis, Student’s t test (two-sided) was used. Results are given as means ± SD.
Fluorescence spectroscopy
The measurements were carried out in black 96-well plates (Nunc) at 25°C using the Cytation 3 microplate reader (BioTek, VT, USA). The final assay volume was 75 µl. Reaction mixtures contained a buffer consisting of 100 µM CaCl2, 100 mM KCl, 5 mM MnCl2, and 75 mM HEPES, pH 7.4. Further, 2-(CI-ANT)-ANPpp compounds, ACT and CaM were added successively. In FRET experiments, 2-(CI-ANT)-ANPpps were used at final concentrations from 10 nM to 500 nM, and ACT and CaM were 300 nM each. Steady-state emission spectra were recorded at low speed with and λex= 280 nm (λem=320–500 nm) and λex= 295 nm (λem = 320–540 nm). In PMEApp displacement experiment, 100 nM 2-(CI-ANT)-ANPpps were displaced from ACT by PMEApp at concentrations of 5 nM to 1 µM. Direct fluorescence of MANTS was excited at 350 nm, and steady-state emission spectra were recorded from 380 to 550 nm. The final concentrations of ACT and CaM were 2.4 µM each. Saturation experiments were performed using MANTs at concentration range from 10 nM to 500 nM; ACT and CaM were 300 nM each. Saturation curves were obtained by subtracting the fluorescence intensity at 430 nm after the addition of ACT from the maximal fluorescence (FRET) after the addition of ACT/CaM. Half-saturation concentration EC50 was calculated using the Graphpad Prism 5 software (San Diego, CA, USA). Basal fluorescence in the presence of buffer alone was subtracted.
Assays with mACs
HEK cells stably expressing AC1, AC2, or AC5 were cultured and frozen as previously described.[41,42] Cells were thawed and plated in white bottom 384-well plates (PerkinElmer, Shelton, CT). Inhibitor compounds (at concentration of 30 µM) were added to cells and incubated at room temperature for 30 min. Then, the specific mAC stimulator (3 µM A23187 for AC1, 100 nM PMA for AC2, and 300 nM forskolin for AC5) in 500 µM 3-isobutyl-1-methyxanthine was added to the cells. Cells were incubated at room temperature for 1 h and cAMP accumulation was measured using Cisbio’s dynamic 2 kit (Cisbio Bioassays, Bedford, MA) according to the manufacturer’s instructions.
Supplementary Material
Acknowledgments
This work was supported by the IOCB research project RVO 61388963, by the Ministry of Interior of the Czech Republic (VG20102015046), Ministry of Education of the Czech Republic (NPU LO1302), Gilead Sciences (Foster City, CA, USA), NIH (MH101673), and Purdue University (West Lafayette, IN, USA).
References
- 1.Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, Mooi FR. Plos Pathog. 2005;1:e45. doi: 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pertussis vaccines: WHO position paper: Weekly Epidemiological Record. 2010;85:385–400. [PubMed] [Google Scholar]
- 3.Kilgore PE, Salim AM, Zervos MJ, Schitt HJ. Clin. Microbiol. Rev. 2016;29:449–486. doi: 10.1128/CMR.00083-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Tiwari T, Murphy TV, Moran J. [acccessed October 2015];Morbidity and Mortality Weekly Report. 2005 Dec 9; http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5414a1.htm. [PubMed]; b) Wood N, McIntyre P. Pediatr. Resp. Rev. 2008;9:201–212. doi: 10.1016/j.prrv.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Bartkus JM, Juni BA, Ehresmann K, Miller CA, Sanden GN, Cassiday PK, Saubolle M, Lee B, Long J, Harrison AR, Jr, Besser JM. J. Clin. Microbiol. 2003;41:1167–1172. doi: 10.1128/JCM.41.3.1167-1172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendelboe AM, Rie AV, Salmaso S, Englund JA. Pediatr. Infect. Dis. J. 2005;24:S58–S61. doi: 10.1097/01.inf.0000160914.59160.41. [DOI] [PubMed] [Google Scholar]
- 7.a) Ladant D, Ullmann A. Trends Microbiol. 1999;7:172–176. doi: 10.1016/s0966-842x(99)01468-7. [DOI] [PubMed] [Google Scholar]; b) Confer DL, Eaton JW. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 8.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Mock M, Ullmann A. Trends Microbiol. 1993;1:187–192. doi: 10.1016/0966-842x(93)90089-a. [DOI] [PubMed] [Google Scholar]; b) Ahuja N, Kumar P, Bhatnagar R. Crit. Rev. Microbiol. 2004;30:187–196. doi: 10.1080/10408410490468795. [DOI] [PubMed] [Google Scholar]; c) Shen Y, Zhukovskaya NL, Zimmer MI, Soelaiman S, Bergson P, Wang CR, Gibbs CS, Tang WJ. Proc. Natl. Acad. Sci. USA. 2004;101:3242–3247. doi: 10.1073/pnas.0306552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Martín C, Gómez-Bilbao G, Ostolaza H. J.Biol. Chem. 2010;285:357–364. doi: 10.1074/jbc.M109.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fisher R, Masin J, Bumba L, Pospisilova E, Fayolle C, Basler M, Sadilkova L, Adkins I, Kamanova J, Cerny J, Konopasek I, Osicka R, Laclerc C, Sebo P. PLoS Pathog. 2012;8(4):e1002580. doi: 10.1371/journal.ppat.1002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Clercq A, Holý E, Rosenberg I, Sakuma T, Balzarini J, Maudgal PC. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 12.a) De Clercq E, Holý A. Nat. Rev. Drug Discovery. 2005;4:928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]; b) De Clercq E. Med. Res. Rev. 2013;33:1278–1303. doi: 10.1002/med.21283. [DOI] [PubMed] [Google Scholar]
- 13.a) Reiser H, Wang J, Chong L, Watkins WJ, Ray AS, Shibata R, Birkus G, Cihlar T, Wu S, Li B, Liu X, Henne IN, Wolfgang GHI, Desai M, Rhodes GR, Fridland A, Lee WA, Plunkett W, Vail D, Thamm DH, Jeraj R, Tumas DB. Clin. Cancer Res. 2008;14:2824–2832. doi: 10.1158/1078-0432.CCR-07-2061. [DOI] [PubMed] [Google Scholar]; b) Zídek Z, Potměšil P, Holý A. Toxicol. Appl. Pharmacol. 2003;192:246–253. doi: 10.1016/s0041-008x(03)00215-1. [DOI] [PubMed] [Google Scholar]
- 14.a) Keough DT, Hocková D, Rejman D, Špaček P, Vrbková S, Krečmerová M, Eng WS, Jans H, West NP, Naesens LM, de Jersey J, Guddat LW. J. Med. Chem. 2013;56:6967–6984. doi: 10.1021/jm400779n. [DOI] [PubMed] [Google Scholar]; b) Eng WS, Hocková D, Špaček P, Janeba Z, West NP, Woods K, Naesens LMJ, Keough DT, Guddat LW. J. Med. Chem. 2015;58:4822–4838. doi: 10.1021/acs.jmedchem.5b00611. [DOI] [PubMed] [Google Scholar]
- 15.a) Keough DT, Hocková D, Holý A, Naesens LM, Skinner-Adams TS, de Jersey J, Guddat LW. J. Med. Chem. 2009;52:4391–4399. doi: 10.1021/jm900267n. [DOI] [PubMed] [Google Scholar]; b) Hocková D, Keough DT, Janeba Z, Wang TH, de Jersey J, Guddat LW. J. Med. Chem. 2012;55:6209–6223. doi: 10.1021/jm300662d. [DOI] [PubMed] [Google Scholar]; c) Keough DT, Špaček P, Hocková D, Tichý T, Vrbková S, Slavětínská L, Janeba Z, Naesens L, Edstein MD, Chavchich M, Wang TH, de Jersey J, Guddat LW. J. Med. Chem. 2013;56:2513–2526. doi: 10.1021/jm301893b. [DOI] [PubMed] [Google Scholar]; d) Keough DT, Hocková D, Janeba Z, Wang T, Naesens L, Edstein MD, Chavchich M, Guddat LW. J. Med. Chem. 2015;58:827–846. doi: 10.1021/jm501416t. [DOI] [PubMed] [Google Scholar]; e) Kaiser MM, Hocková D, Wang T-H, Draèínský M, Poštová-Slavìtínská L, Procházková E, Edstein MD, Chavchich M, Keough DT, Guddat LW, Janeba Z. ChemMedChem. 2015;10:1707–1723. doi: 10.1002/cmdc.201500322. [DOI] [PubMed] [Google Scholar]
- 16.a) Zídek Z, Franková D, Holý A. Antimicrob. Agents Chemother. 2001;45:3381–3386. doi: 10.1128/AAC.45.12.3381-3386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zídek Z, Franková D, Holý A. Int. J. Immunopharmacol. 2000;22:1121–1129. doi: 10.1016/s0192-0561(00)00068-0. [DOI] [PubMed] [Google Scholar]; c) Potměšil P, Krečmerová M, Kmoníčková E, Holý A, Zídek Z. Eur. J. Pharmacol. 2006;540:191–199. doi: 10.1016/j.ejphar.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Guo Q, Shen Y, Lee Y-S, Gibbs CS, Mrksich M, Tang W. EMBO J. 2005;24:3190–3201. doi: 10.1038/sj.emboj.7600800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen YQ, Zhukovskaya NL, Zimmer MI, Soelaiman S, Bergson P, Wang CR, Gibbs CS, Tang WJ. Proc. Natl. Acad. Sci. USA. 2004;101:3242–3247. doi: 10.1073/pnas.0306552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoshani I, Laux WHG, Périgaud Ch, Gosselin G, Johnson RA. J. Biol. Chem. 1999;274:34742–34744. doi: 10.1074/jbc.274.49.34742. [DOI] [PubMed] [Google Scholar]
- 20.Česnek M, Jansa P, Šmídková M, Mertlíková-Kaiserová H, Dračínský M, Brust TF, Pávek P, Trejtnar F, Watts VJ, Janeba Z. ChemMedChem. 2015;10:1351–1364. doi: 10.1002/cmdc.201500183. [DOI] [PubMed] [Google Scholar]
- 21.a) Šmídková M, Dvořáková A, Tloušťová E, Česnek M, Janeba Z, Mertlíková-Kaiserová H. Antimicrob. Agents Chemother. 2014;58:664–671. doi: 10.1128/AAC.01685-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pradere U, Garnier-Amblard EC, Coats SJ, Amblard F, Schinazi RF. Chem. Rev. 2014;114:9154–9218. doi: 10.1021/cr5002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a) Gille A, Seifert R. J. Biol. Chem. 2003;278:12672–12679. doi: 10.1074/jbc.M211292200. [DOI] [PubMed] [Google Scholar]; b) Gille A, Lushington GH, Mou TC, Doughty MB, Johnson RA, Seifert R. J. Biol. Chem. 2004;279:19955–19969. doi: 10.1074/jbc.M312560200. [DOI] [PubMed] [Google Scholar]; c) Seifert R, Dove S. Trends Microbiol. 2012;20:343–351. doi: 10.1016/j.tim.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Hiratsuka T. J. Biol. Chem. 1985;260:4784–4790. [PubMed] [Google Scholar]
- 24.Göttle M, Dove S, Steindel P, Shen Y, Tang W, Geduhn J, König B, Seifert R. Mol. Pharmacol. 2007;72:526–535. doi: 10.1124/mol.107.034413. [DOI] [PubMed] [Google Scholar]
- 25.Holý A. Collect. Czech. Chem. Commun. 1978;43:2054–2031. [Google Scholar]
- 26.a) Kuhn K, Owen DJ, Bader B, Wittinghofer A, Kuhlmann J, Waldmann H. J. Am. Chem. Soc. 2001;123:1023–1035. doi: 10.1021/ja002723o. [DOI] [PubMed] [Google Scholar]; b) Hradilová L, Poláková M, Dvořáková B, Hajdúch M, Petruš L. Carboh. Res. 2012;361:1–6. doi: 10.1016/j.carres.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Chen JJ, Cai X, Szostak JW. J. Am. Chem. Soc. 2009;131:2119–2121. doi: 10.1021/ja809069b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.a) Allison BD, Phuong VK, McAtee LC, et al. J. Med. Chem. 2006;49:6371–6390. doi: 10.1021/jm060590x. [DOI] [PubMed] [Google Scholar]; b) Twin H, Batey RA. Org. Lett. 2011;6:4913–4916. doi: 10.1021/ol0479848. [DOI] [PubMed] [Google Scholar]; c) Liu J, Deng X, Fitzgerald AE, Sales ZS, Vankatesan H, Mani S. Org. Biomol. Chem. 2011;9:2654–2660. doi: 10.1039/c0ob01004a. [DOI] [PubMed] [Google Scholar]
- 29.a) Krečmerová M, Masojídková M, Holý A. Collect. Czech. Chem. Commun. 2004;69:1889–1913. [Google Scholar]; b) Krečmerová M, Dračínský M, Hocková D, Holý A, Keough DT, Guddat LW. Bioorg. Med. Chem. 2012;20:1222–1230. doi: 10.1016/j.bmc.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Vrbovská S, Holý A, Pohl R, Masojídková M. Collect. Czech. Chem. Commun. 2006;71:543–566. [Google Scholar]
- 31.Jansa P, Baszczynski O, Dračínský M, Votruba I, Zídek Z, Bahador G, Stepan G, Cihlar T, Mackman R, Holý A, Janeba Z. Eur. J. Med. Chem. 2011;46:3748–3754. doi: 10.1016/j.ejmech.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 32.a) Holý A, Rosenberg I. Collect. Czech. Chem. Commun. 1987;52:2801–2809. [Google Scholar]; b) Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1961;83:649–658. [Google Scholar]
- 33.Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, Cattaneo M, Zighetti ML, Chen A, Kim S, Kim HS, Bischofberger N, Cook G, Jacobson KA. J. Med. Chem. 2002;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HS, Barak D, Harden TK, Boyer JL, Jacobson KA. J. Med. Chem. 2001;44:3092–3108. doi: 10.1021/jm010082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hackett M, Walker CB, Guo L, Gray MC, Van Cuyk S, Ullmann A, Shabonowith J, Hunt DF, Hewlett EL, Sebo P. J. Biol. Chem. 1995;270:20250–20253. doi: 10.1074/jbc.270.35.20250. [DOI] [PubMed] [Google Scholar]
- 36.Iwaki M, Kamachi K, Konda T. Infect. Immun. 2000;68:3727–3730. doi: 10.1128/iai.68.6.3727-3730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkus G, Wang R, Liu X, Kutty N, MacArthur H, Cihlar T, Gibbs C, Swaminathan S, Lee W, McDemott M. Antimicrob. Agents Chemother. 2007;51:543–550. doi: 10.1128/AAC.00968-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luedtke CC, Andonian S, Igdoura S, Hermo L. J. Histochem. Cytochem. 2000;48:1131–1146. doi: 10.1177/002215540004800810. [DOI] [PubMed] [Google Scholar]
- 39.Molecular Operating Environment (MOE) 2014:0901. [Google Scholar]; Chemical Computing Group Inc. 1010 Sherbooke St. West, Suite #910. Montreal, QC, Canada, H3A 2R7: 2016. [Google Scholar]
- 40.Šolínová V, Kaiser MM, Lukáč M, Janeba Z, Kašička V. J. Sep. Sci. 2014;37:295–303. doi: 10.1002/jssc.201301092. [DOI] [PubMed] [Google Scholar]
- 41.Conley JM, Brand CS, Bogard AS, Pratt EPS, Xu RQ, Hockerman GH, Ostrom RS, Dessauer CW, Watts VJ. J. Pharm. and Exp. Ther. 2013;347:276–287. doi: 10.1124/jpet.113.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conley JM, Brust TF, Xu R, Burris KD, Watts VJ. J. Vis. Exp. 2014;83:e51218. doi: 10.3791/51218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.