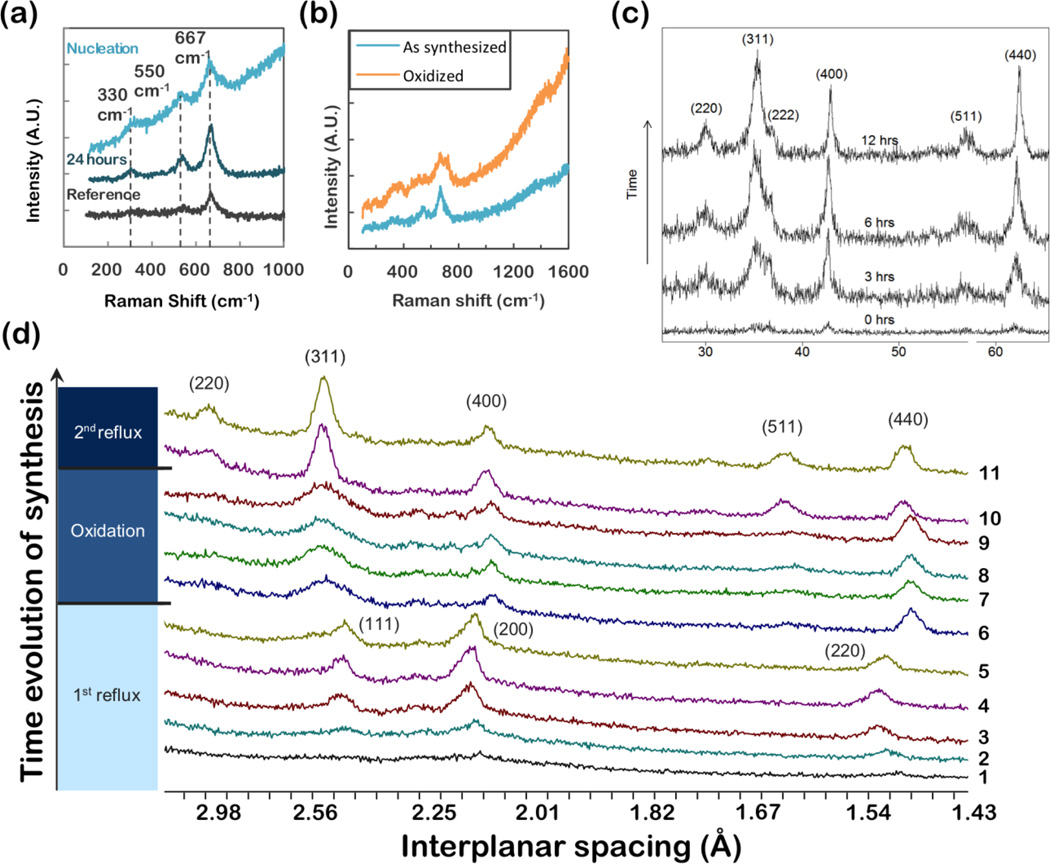
Figure 6.
(a) Raman spectroscopy of iron oxide nanoparticles synthesized from iron(III) oleate and characterized at nucleation and after 24 hours of aging. (b) Synthesized from FeOOH, and oxidized to maghemite (c) XRD, θ - 2θ scans, of iron oxide nanoparticles synthesized form FeOOH. Particles were annealed at 100°C for various times to optimize phase and crystallinity. The peaks observed on annealing can be readily indexed as magnetite. (d) XRD of iron oxide nanoparticles produced by thermal decomposition of Fe(CO)5. Aliquots were taken throughout the synthesis (§2.1.1), and during subsequent oxidation (§2.1.4). Particles initially form as wüstite (FeO1-x), as indicated by the (111), (200) and (220) wüstite peaks in aliquots 1–5. During oxidation (aliquots 6–9) magnetite peaks clearly develop. A second reflux step (aliquots 10 and 11) optimizes the crystallinity, as indicated by sharpening of peaks.