Figure 5. Metaeffector activity through deubiquitination.
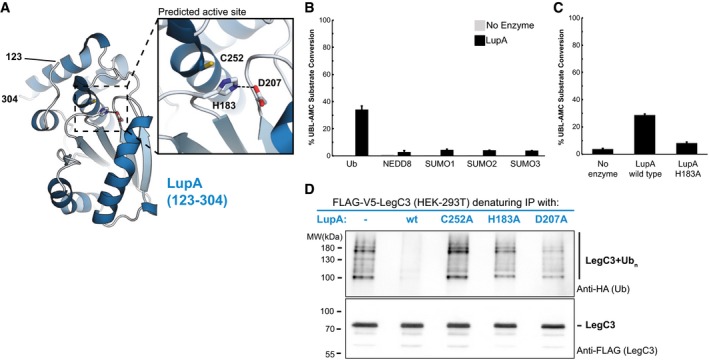
- The de novo crystal structure of the metaeffector LupA (123–304) was determined to 1.9 Å resolution and reveals that LupA belongs to the ubiquitin or ubiquitin‐like protease (UBP) family of proteins with a canonical cysteine protease triad (inset).
- LupA displays deubiquitinase activity in vitro. A fluorescence‐based assay was used to monitor the catalytic hydrolysis of ubiquitin and ubiquitin‐like proteins from a covalently linked fluorophore (AMC) after incubation with purified LupA or a no‐enzyme control. Substrates tested are displayed on the x‐axis, and % substrate conversion on the y‐axis. LupA activity is specific toward ubiquitin. The activity of LupA against ubiquitin‐AMC is significantly different than its activity against each of the other ubiquitin‐like substrates as assessed by unpaired, two‐tailed Student's t‐tests (Ub versus Nedd8: P‐value = 0.005; SUMO‐1: P‐value = 0.005; SUMO‐2: P‐value = 0.005; SUMO‐3: P‐value = 0.005; n = 2). The error bars indicate the SD.
- Mutation of a predicted catalytic residue (H183) almost completely abolishes the in vitro hydrolase activity. In the fluorescence‐based assay described above, the mutant activity is significantly reduced from the wild‐type enzyme activity as assessed by an unpaired, two‐tailed Student's t‐test (P‐value = 0.000009, n = 3). The error bars indicate the SD.
- LupA activity removes polyubiquitin linkages from its cognate IDTS, LegC3. Denaturing IPs of FLAG‐V5‐LegC3 expressed in HEK293T cells were analyzed by western blot and probed for ubiquitination during co‐expression of V5‐LupA or one of three catalytically impaired variants (see Appendix Fig S6C for input). While polyubiquitination of LegC3 was present when co‐expressed with a catalytically inactive LupA (or in the absence of LupA), no ubiquitination could be detected in the presence of wild‐type LupA, confirming its deubiquitination of LegC3 in vivo.
