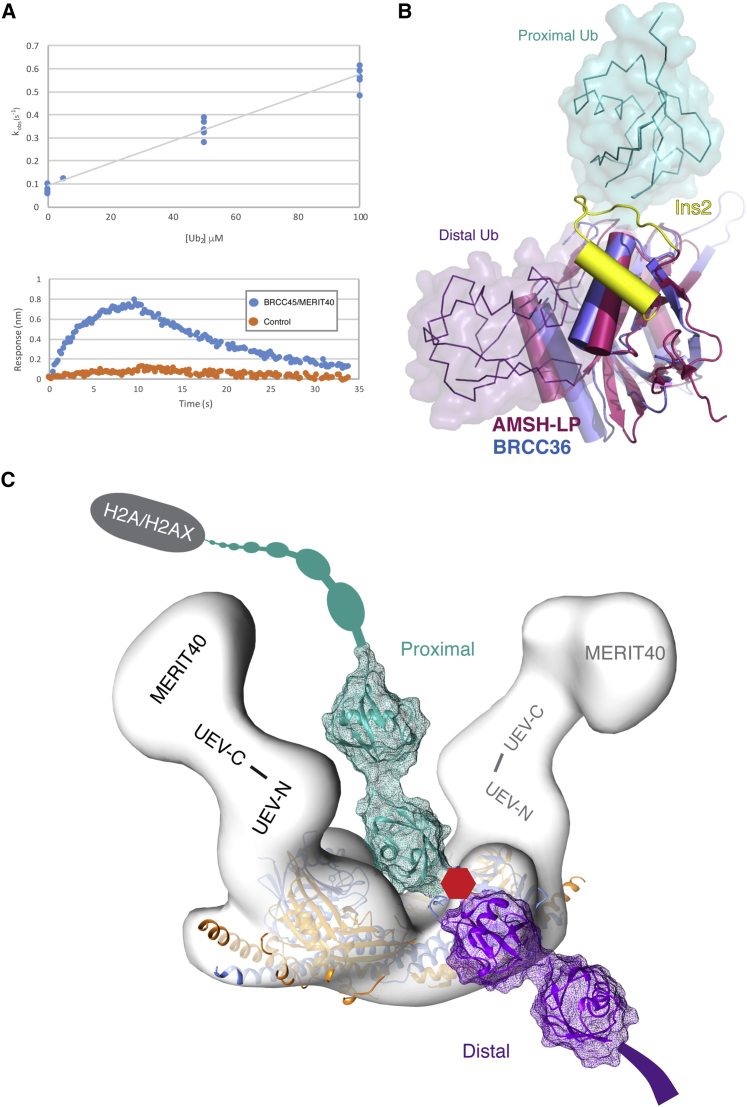
Figure 3.
Ubiquitin Binding by BRCA1-A
(A) Upper panel: binding of di-ubiquitin to the BRCC45/MERIT40 subcomplex measured by biolayer interferometry. The top panel shows the pseudo first-order association constant (Kobs) plotted against K63-linked Ub2 concentration. The dissociation equilibrium constant (Kd) of 17 uM was determined from the ratio of koff and kon. Lower panel: association and dissociation phases in the raw kinetic trace for Ub2 binding to a probe linked to the BRCC45/MERIT40 complex (blue) and a non-derivatized probe (orange).
(B) Superposition of the AMSH-LP/K63-Ub2 complex with the docked Abro1/BRCC36 structure shows the approximate positions of the proximal (teal) and distal (purple) Ub molecules. As previously noted (Zeqiraj et al., 2015), the Ins-2 loop that packs against the proximal Ub in AMSH-LP (yellow) is absent in BRCC36.
(C) The alignment in (A) and the structure of K63-linked di-ubiquitin were used to model an extended Ub chain on the proximal and distal sides of the cleavage site into the 2×4 complex (white surface). Abraxas and BRCC36 are shown in gold and blue, respectively.