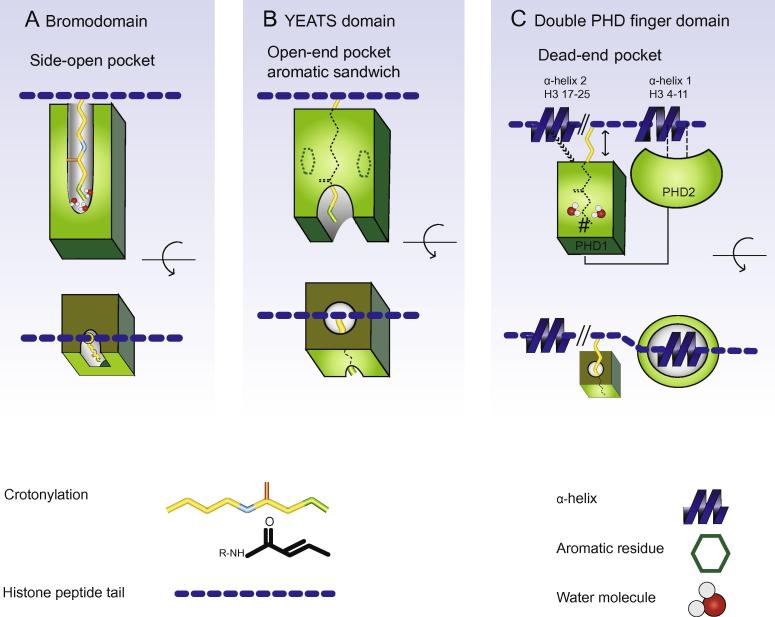
Figure 1.
Schematic overview of the recognition modes of crotonyl-lysine by the bromodomain, YEATS domain, and double PHD finger domainA. The bromodomain (BDR) adopts a side-open pocket for recognition of histone acylations. Four bundled left-handed helices and two inter-helical loops constitute an aromatic, hydrophobic pocket for binding to a wide range of histone acetylations. However, only the BDR of Taf1(2) accommodates large acylations like Kcr, because it is capable of rearranging the water network deep within the pocket (indicated with water molecules). Other BDRs cannot accommodate Kcr in co-planar fashion, and fail to bind to them with high affinity. B. The YEATS domain is an open-end pocket that binds to Kcr with higher affinity than Kac by means of aromatic stacking [6]. C. The double PHD fingers of MOZ interact with each other and cooperatively bind to H3K14ac. PHD2 induces an α-helix at H3K4-T11 [22], while H3 and K4 (indicated with dashed lines) interact with the β1 of PHD2. PHD1 induces second α-helix and forms a binding pocket for Kcr, allowing H3L20 and K23 to associate with the so-called I128A and L213A saddle pair of β2 (arrow headed line). DPF-acetyl-lysine recognition depends on a tight fit, brought about by intimate hydrophobic encapsulation (indicated with water molecules) and coordinated hydrogen bonding. This generates a larger pocket and specific interactions that favor Kcr. However, even though residues with small side chains circumvent steric hindrance and allow co-planer insertion of Kcr within the β2 pocket (approximated with the pound sign), the large acylations still stick out of the dead-ended reading pocket (double-arrow headed line). Surprisingly, this does not compromise binding affinity.