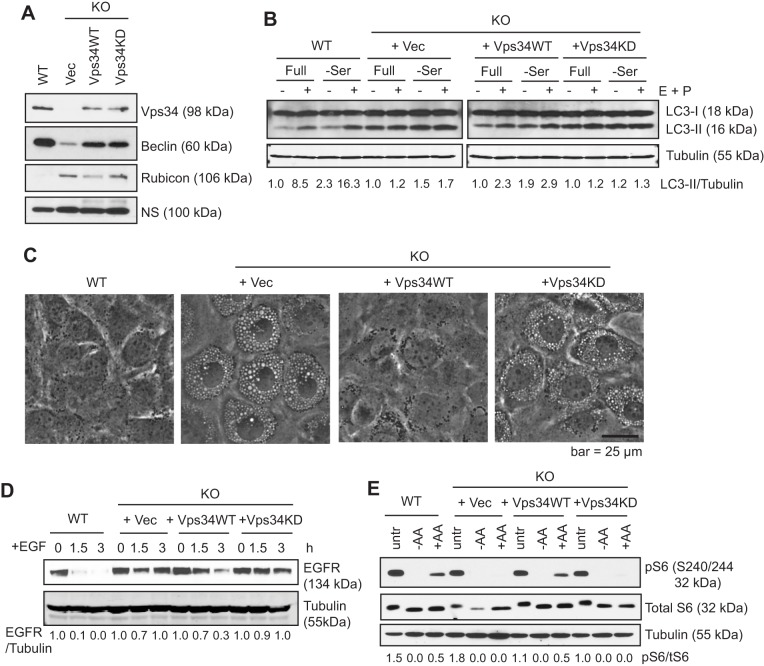
Fig. 1.
Vps34 functions are dependent on its catalytic activity. (A) Empty vector (Vec), HA-tagged Vps34 wild-type (Vps34WT) or Vps34 kinase-dead mutant K771A (Vps34KD) were stably expressed in Vps34−/− (KO) MEFs. The indicated proteins were probed through immunoblotting. NS, non-specific band. (B) Vps34WT but not Vps34KD rescues autophagic flux. The indicated MEFs were cultured in full or serum-free medium for 6 h, with or without 10 μg/ml E64D and 10 μg/ml Pepstatin A (E+P) to block lysosomal degradation. Autophagic flux is evaluated by comparing the levels of the lipidated form of LC3 (LC3-II) and tubulin (LC3-II/tubulin) in the presence and absence of the protease inhibitors using ImageJ. Results are a quantification of the experiment shown. (C) Intracellular vacuolization induced in Vps34−/− cells is rescued by wild-type but not kinase-dead Vps34. The indicated cells were photographed and representative images are shown. (n=3). (D) EGFR degradation is rescued by wild-type but not kinase-dead Vps34. The indicated cells were serum-starved overnight and then stimulated with 100 ng/ml EGF for the indicated times. The level of EGF receptor was analyzed by immunoblotting. The relative level of EGFR compared with tubulin of a representative experiment of two independent experiments is shown. (E) Amino-acid-induced mTOR signaling is rescued by wild-type but not kinase-dead Vps34. The indicated cells were left untreated (untr), amino acid starved (−AA), or starved and re-stimulated with 2× the amount of amino acids in MEM for 30 min (+AA). Cell lysates were probed with antibodies against the ribosomal protein S6 or its phosphorylated form (pS6) by immunoblotting. The ratio of pS6 to total S6 of a representative experiment (of two independent experiments) is shown.