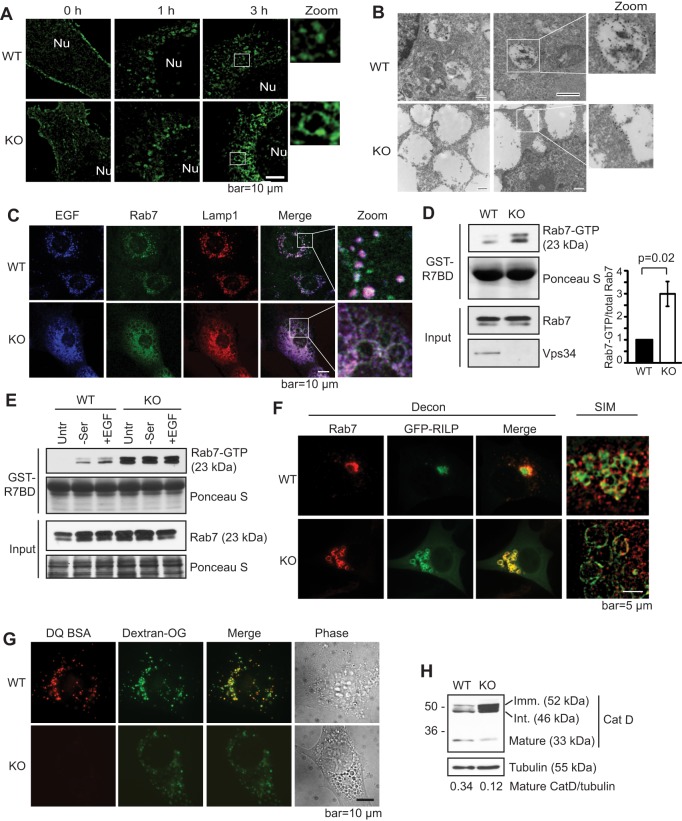
Fig. 3.
Knockout of Vps34 leads to Rab7 hyperactivation, late endosome enlargement and defective lysosomal function. (A) Internalized EGFR localizes to the limiting membrane of enlarged endosomes in Vps34−/− cells. GFP–EGFR was expressed in Vps34+/+ (WT) and Vps34−/− (KO) MEFs. Cells were serum starved and stimulated with 100 ng/ml EGF for the indicated times. The GFP–EGFR localization was analyzed by super-resolution SIM. The boxed areas are shown at a higher magnification (zoom). Nu, nucleus. (n=4). (B) GFP–EGFR was transfected into Vps34+/+ or Vps34−/− MEFs. Cell were starved overnight, then left untreated or stimulated with 100 ng/ml EGF in serum-free media for 1.5 h. Immuno-gold electron microscopy against anti-GFP reveals the accumulation of GFP–EGFR in the lumen of endosomes in Vps34+/+ but on the limiting membrane of enlarged endosomes in Vps34−/− cells. Two representative cells of each cell type are shown. The boxed areas are further magnified to help visualize gold particles. (C) EGF accumulates on the limiting membrane of enlarged late endosomes in Vps34−/− cells. Vps34+/+ and Vps34−/− MEFs were stimulated with EGF–Alexa-Fluor-647 for 45 min. Cells were stained for endogenous Rab7 and Lamp1, and analyzed by confocal microscopy. (D) Rab7-GTP levels are increased in Vps34−/− MEFs. A Rab7 activity assay was performed using the Rab7-binding domain (R7BD) of RILP fused to GST, which pulls down GTP-bound Rab7. Shown on the right is the relative amount of Rab7-GTP as the mean±s.d. from three independent experiments. The P-value was calculated with a Student's t-test. (E) Vps34−/− cells have an elevated Rab7-GTP level that is refractory to starvation and EGF stimulation. Vps34+/+ and Vps34−/− MEFs were left untreated (untr), serum starved (−ser), or serum starved and then stimulated with 100 ng/ml EGF stimulation (+EGF). A Rab7 activity assay was performed using GST–R7BD. (F) The Rab7 effector RILP localizes on the limiting membrane of enlarged late endosomes in Vps34−/− cells. GFP–RILP was transfected in Vps34+/+ and Vps34−/− cells. Cells were fixed and immunostained for endogenous Rab7. Cells were observed under deconvolution or SIM microscopes. Note that RILP overexpression causes perinuclear clustering of late endosomes in wild-type cells, as expected, and that Rab7 and RILP colocalize on enlarged late endosome structures. (G) Lysosomal protease activity is diminished in Vps34−/− MEFs. Cells were loaded with 10 μg/ml Dextran Oregon Green for 16 h, subjected to a 4-h chase, then loaded with DQ-BSA for 1 h. Cells were washed extensively and the fluorescence pattern was analyzed in live cells using deconvolution microscope. Note that DQ-BSA cleavage is severely compromised in Vps34−/− cells (n=3). (H) Vps34 deletion disrupts cathepsin D maturation. The steady-state levels of immature (52 kDa), intermediate (46 kDa) and mature (33 kDa) cathepsin D were analyzed by immunoblotting (n=4).