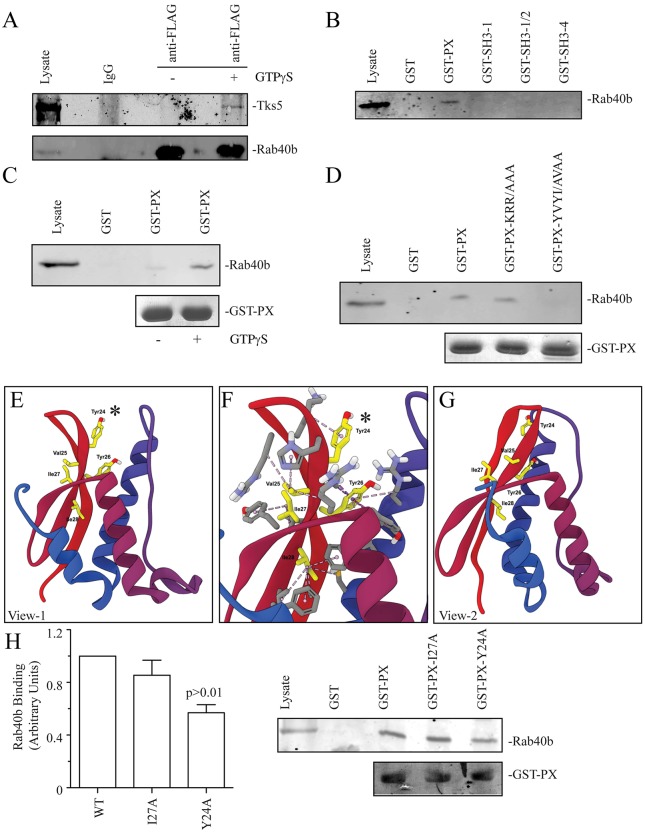
Fig. 4.
Rab40b binds Tks5-PX domain. (A) MDA-MB-231 cells stably expressing FLAG–Rab40b were lysed and lysates incubated in the absence or presence of GTPγS. FLAG–Rab40b was then immunoprecipitated using anti-FLAG antibody and immunoblotted with an anti-Tks5 antibody. Purified non-specific mice IgG was used as a control in immunoprecipitation experiments. (B) Glutathione bead pulldown assays with various GST-tagged Tks5 fragments. (C) GST–Tks5-PX (GST-PX)-coated glutathione beads were incubated with MDA-MB-231 lysates in the presence or absence of GTPγS. The amount of bound FLAG–Rab40b was then analyzed by immunoblotting with anti-FLAG antibodies. (D) Glutathione bead pulldown assays with various Tks5-PX mutants. (E–G) Models of PX domain structure depicting the position of residues and hydrophobic interactions within the PX domain. E and G show PX domains from different sides; F shows a magnified view of E that includes the 23-YVUI-28 motif. The asterisk highlights Tyr24, which is required for Rab40b binding. (H) The effect of Y27A and Y24A mutations on the ability of GST–PX to bind FLAG–Rab40b. Data shown are the mean±s.d. derived from three independent experiments.