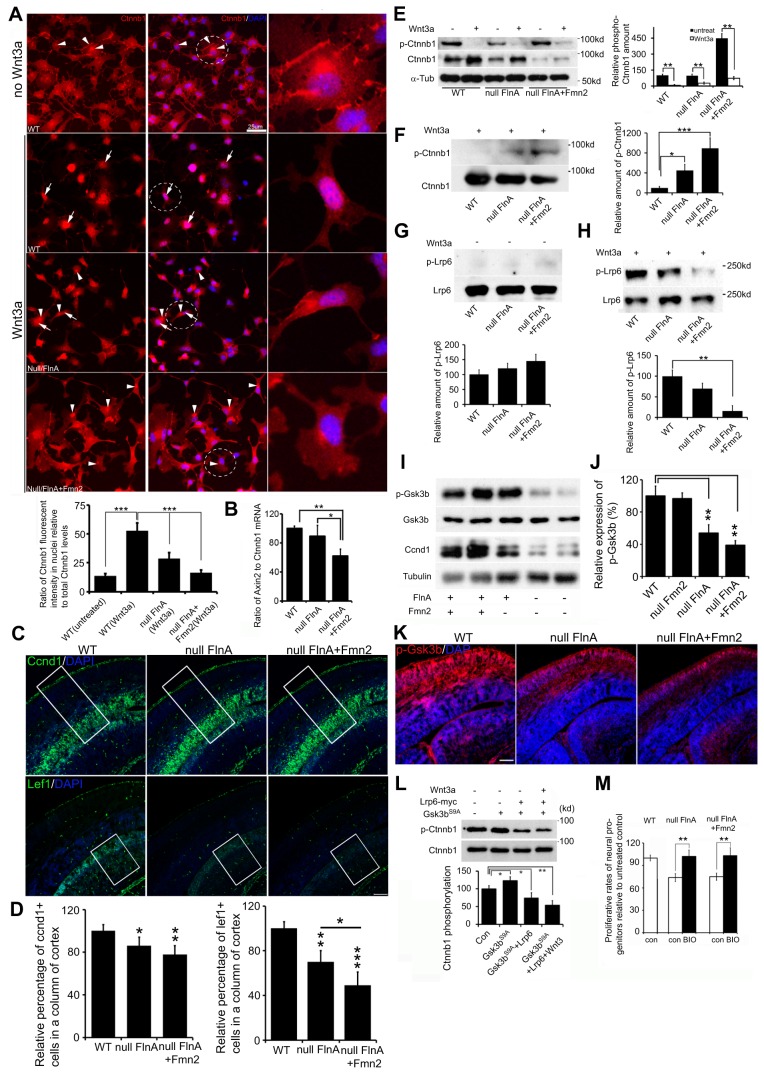
Fig. 7.
Loss of FlnA and Fmn2 impairs Wnt downstream signaling transduction. (A) Loss of FlnA and Fmn2 impairs Wnt3a-stimulated nuclear accumulation of β-catenin. Neural progenitor cells were treated with Wnt3a for 3-6 h, and then stained with anti-β-catenin and counterstained with DAPI. β-catenin was localized in the cytosol and at the cytoplasmic membrane in untreated neural progenitor cells (only WT cell staining is shown). Nuclear accumulation of β-catenin was increased in WT cells but not in Flna null and Flna+Fmn2 null cells after Wnt3a treatment. Statistical analysis (from three repeat assays) showed that the fluorescence intensity of β-catenin in nuclei of Flna+Fmn2 null cells was significantly lower than in WT and Flna null cells. n>12 samples were used for each time point. (B) Digital PCR shows that the Axin2 transcript, a Wnt downstream target, is significantly decreased in Flna+Fmn2 null neural progenitors compared with Flna null and WT cells, indicating diminished Wnt signaling activity in Flna+Fmn2 null neural progenitors. (C) Fluorescent photomicrographs showing that the number of Ccnd1+ cells and Lef1+ cells in Flna+Fmn2 null E15.5 cortex is reduced compared with littermate WT and Flna null cortices. (D) Statistical analyses show that the number of Ccnd1+ cells in Flna+Fmn2 null cortex is reduced by about 6% and 20% and the number of Lef1+ cells is reduced by 20% and 50%, as compared with Flna null and WT cortices, respectively. (E) Wnt3a stimulation promotes β-catenin expression in WT and Flna null neural progenitor cells and inhibits β-catenin phosphorylation in these cells. Statistical analyses (right) show that relative amounts of phospho-β-catenin are significantly decreased after Wnt3a stimulation. (F) Western blot demonstrates a decrease in phospho-β-catenin in WT control neural cells following Wnt3a stimulation. The decrease in β-catenin phosphorylation is impaired in Flna null cells and more so in Flna+Fmn2 null cells. Results are quantified (right) from n>3 independent assays. (G) Western blot demonstrates no significant difference in phospho-Lrp6 following loss of FlnA or of FlnA and Fmn2 compared with WT in the absence of Wnt3a stimulation. Results are quantified (beneath) from n>3 independent assays. (H) Western blot shows impaired Lrp6 phosphorylation after Wnt3a stimulation in the Flna null and Flna+Fmn2 null neural cells. Results are quantified (beneath) from n>3 independent assays. (I) Western blot shows that Gsk3β phosphorylation (Ser9) levels in Flna null and Flna+Fmn2 null brains are decreased. The expression of Ccnd1 is also diminished in the brain lysates. (J) Statistical analyses show that the levels of phospho-Gsk3β in Flna null and Flna+Fmn2 null brains are significantly decreased. (K) Fluorescent photomicrographs of E15.5 brains following immunostaining show progressively reduced phospho-Gsk3β (pSer9) levels (Rhodamine) in Flna null and Flna+Fmn2 null cortices. Nuclei are counterstained with DAPI. (L) Lrp6 overexpression inhibits Gsk3βS9A phosphorylation of β-catenin. Gsk3βS9A is a constitutively active, non-phosphorylated Gsk3β with serine to alanine substitution. Statistical analysis (n>3) shows that Lrp6 expression significantly decreases Gsk3βS9A phosphorylation of β-catenin, and Wnt3a (200 ng/ml) stimulation further increases the inhibitory effect of Lrp6 on β-catenin phosphorylation. (M) Treatment with the Gsk3β inhibitor BIO rescues the proliferation of Flna null and Flna+Fmn2 null neural progenitor cells. Proliferative rates for Flna null or Flna+Fmn2 null neural progenitors are reduced by ∼25% compared with WT control. BIO treatment leads to recovery in the proliferative rates of both Flna null and Flna+Fmn2 null neural progenitor cells, to a level comparable to that seen in untreated WT cells. Neural progenitor cells were cultured in NPC medium with or without 1 μM BIO for 48 h, and total cell numbers were determined by cell counting. ***P<0.001, **P<0.01, *P<0.05, two-tailed t-test. Error bars indicate s.d. Scale bars: 10 μm in A; 100 μm in C,K.