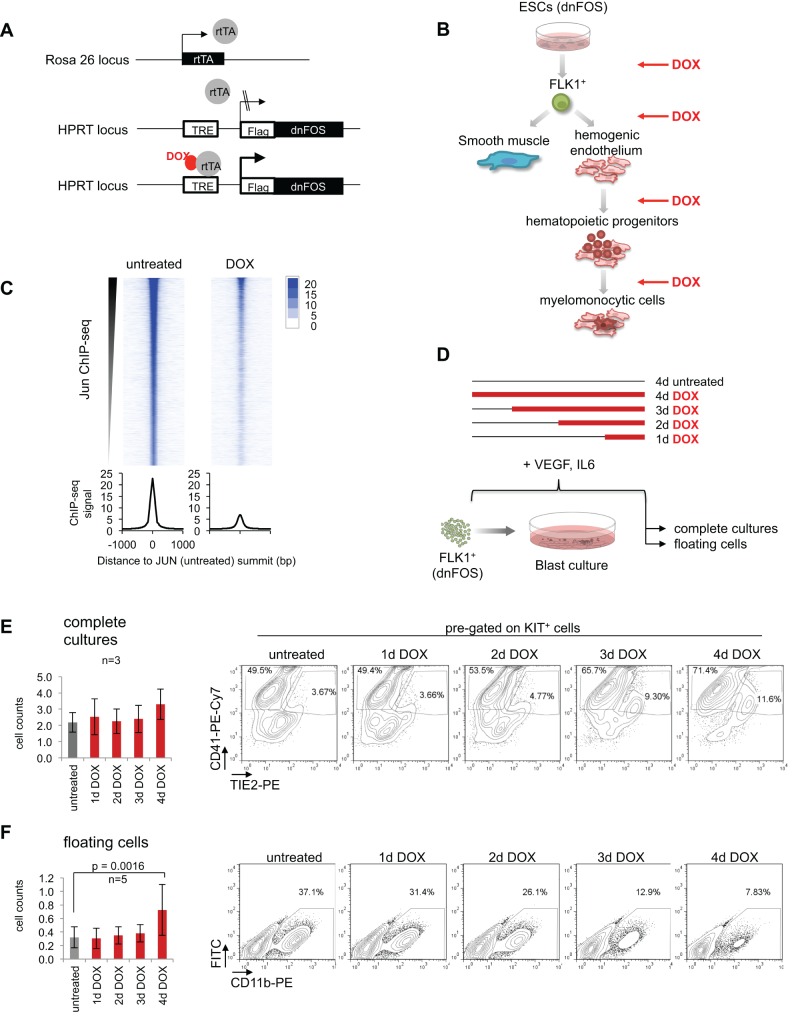
Fig. 1.
AP-1 inhibition affects differentiation of ESCs to hemangioblast, to hemogenic and to hematopoietic cells. (A) Schematic representation of the targeted HPRT-gene locus of the DOX-inducible dnFOS-expressing A17 2lox mouse ESC line (dnFOS ESCs). (B) Overview of the in vitro differentiation of ESCs to blood cells and the corresponding time course of dnFOS induction (DOX). (C) JUN ChIP-seq in uninduced and DOX-treated dnFOS cells. FLK1+ dnFOS cells were cultured under blast culture conditions for 1 day±1 µg/ml DOX. Cells of complete cultures were double crosslinked and chromatin was used for JUN ChIP followed by genome-wide sequencing. The JUN ChIP-seq signal in the untreated dataset and the corresponding signal detected in the DOX dataset are shown. (D) Schematic overview of the DOX-induction time course approach during blast culture. FLK1+ dnFOS cells were cultured for 4 days under blast culture conditions. Cells were either left untreated (4 days untreated) or DOX was added from start of culture (for 4 days=4 days DOX), at day 1 (3 days DOX), day 2 (2 days DOX) or day 3 (1 day DOX) of culture. At day 4, complete cultures and floating cells were analysed for cell numbers and surface marker profiles by flow cytometry. (E) Total cell counts (left) and a representative CD41-/Tie2-specific flow cytometric analysis of pre-gated cKitpos cells (right) of day 4 complete blast cultures that were either left untreated or DOX-treated for 1 day, 2 days, 3 days or 4 days (data are mean±s.d., n=3). (F) Total cell counts (left) and a representative CD11b-specific flow cytometric analysis (right) of day 4 floating cells derived from dnFOS blast cultures that were either left untreated or DOX treated for 1 day, 2 days, 3 days or 4 days (data are mean ±s.d., n=3).