Abstract
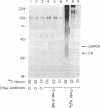
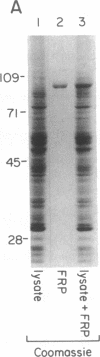
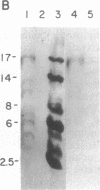
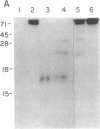
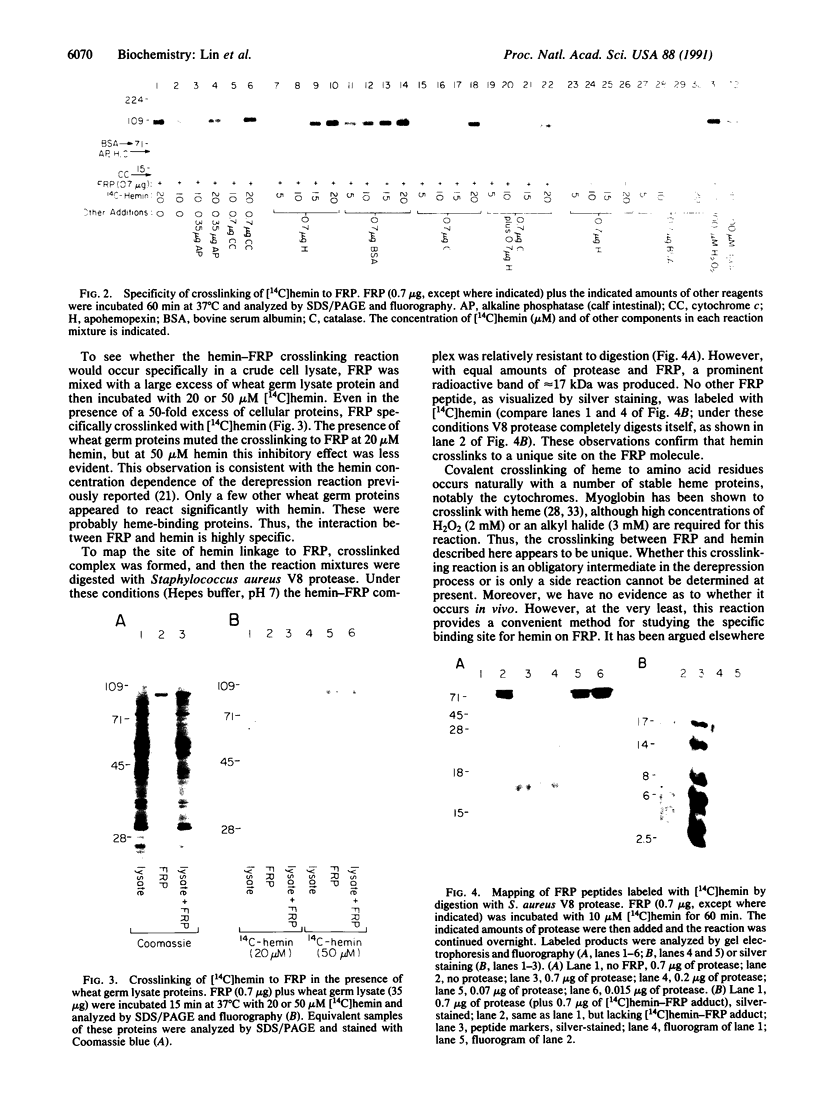
Incubation of a 90-kDa ferritin repressor protein (FRP) with small amounts of radiolabeled hemin resulted in the formation of a strong interaction between the two that was stable to SDS/PAGE. (We refer to this interaction as a "crosslink," without intending to imply knowledge as to its chemical nature.) Of seven other proteins tested individually, only apohemopexin and bovine serum albumin showed similar crosslinking ability, albeit to a much lower extent. [14C]Hemin specifically crosslinked to FRP in the presence of a 50-fold excess of total wheat germ proteins. Inclusion of catalase did not prevent the reaction of hemin with FRP, suggesting that H2O2 is not involved. The subsequent addition of a stoichiometric amount of apohemopexin did not reverse the reaction. Exhaustive digestion of the complex with Staphylococcus aureus V8 protease produced a major labeled peptide of 17 kDa. These results show the existence of a highly specific, uniquely reactive hemin binding site on FRP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aft R. L., Mueller G. C. Hemin-mediated oxidative degradation of proteins. J Biol Chem. 1984 Jan 10;259(1):301–305. [PubMed] [Google Scholar]
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Daniels-McQueen S., Walden W. E., Patino M. M., Gaffield L., Bielser D., Thach R. E. Requirements for the translational repression of ferritin transcripts in wheat germ extracts by a 90-kDa protein from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13383–13386. [PubMed] [Google Scholar]
- Catalano C. E., Choe Y. S., Ortiz de Montellano P. R. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J Biol Chem. 1989 Jun 25;264(18):10534–10541. [PubMed] [Google Scholar]
- Chu L. L., Fineberg R. A. On the mechanism of iron-induced synthesis of apoferritin in HeLa cells. J Biol Chem. 1969 Jul 25;244(14):3847–3854. [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Gaffield L., Patino M. M., Walden W. E., Thach R. E. Specificity of the induction of ferritin synthesis by hemin. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):146–150. doi: 10.1016/0167-4781(90)90156-v. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Patino M. M., Gaffield L., Walden W. E., Thach R. E. Derepression of ferritin messenger RNA translation by hemin in vitro. Science. 1990 Jan 5;247(4938):74–77. doi: 10.1126/science.2294594. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Murray M. T., White K., Munro H. N. Conservation of ferritin heavy subunit gene structure: implications for the regulation of ferritin gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7438–7442. doi: 10.1073/pnas.84.21.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y., Martin B. M., Griffin P. R., Yates J. R., 3rd, Shabanowitz J., Hunt D. F., Murphy A. C., Chen L., Cotter R. J., Pohl L. R. Metabolism-based covalent bonding of the heme prosthetic group to its apoprotein during the reductive debromination of BrCCl3 by myoglobin. J Biol Chem. 1990 Jun 25;265(18):10340–10346. [PubMed] [Google Scholar]
- Rotenberg M., Margalit R. Deuteroporphyrin-albumin binding equilibrium. The effects of porphyrin self-aggregation studied for the human and the bovine proteins. Biochem J. 1985 Jul 1;229(1):197–203. doi: 10.1042/bj2290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Sinclair P. R., Bement W. J., Gorman N., Liem H. H., Wolkoff A. W., Muller-Eberhard U. Effect of serum proteins on haem uptake and metabolism in primary cultures of liver cells. Biochem J. 1988 Nov 15;256(1):159–165. doi: 10.1042/bj2560159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson G. R., Patino M. M., Beck M. M., Gaffield L., Walden W. E. Characteristics of the interaction of the ferritin repressor protein with the iron-responsive element. Biol Met. 1991;4(1):48–55. doi: 10.1007/BF01135557. [DOI] [PubMed] [Google Scholar]
- Tew D., Ortiz de Montellano P. R. The myoglobin protein radical. Coupling of Tyr-103 to Tyr-151 in the H2O2-mediated cross-linking of sperm whale myoglobin. J Biol Chem. 1988 Nov 25;263(33):17880–17886. [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Vincent S. H., Grady R. W., Shaklai N., Snider J. M., Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch Biochem Biophys. 1988 Sep;265(2):539–550. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- Vincent S. H., Muller-Eberhard U. Effects of porphyrins on proteins of cytosol and plasma. In vitro photo-oxidation and cross-linking of proteins by naturally occurring and synthetic porphyrins. J Lab Clin Med. 1987 Oct;110(4):475–482. [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]