Figure 6.
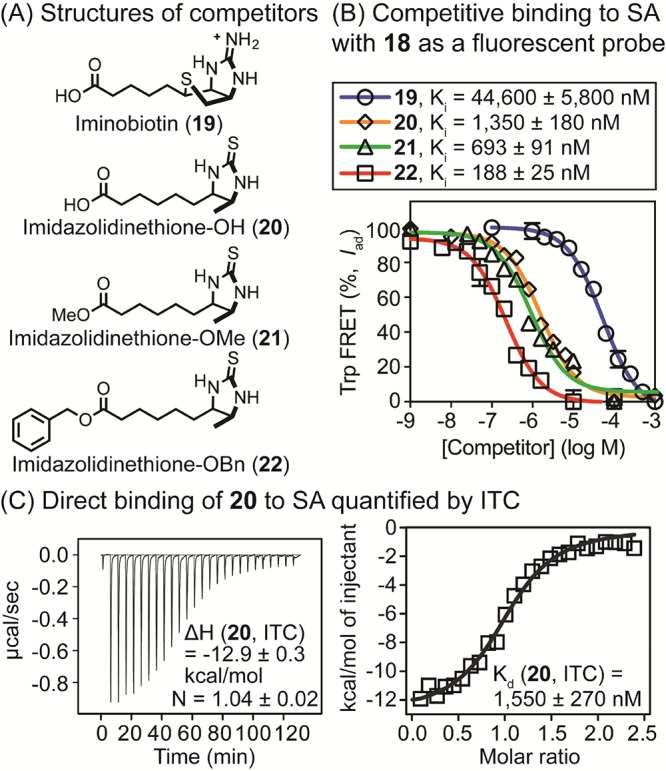
(A) Structures of nonfluorescent analogues of biotin (19–22) used in competition binding assays. (B) Quantification of competitive inhibitory constants (Ki) of 19–22 for SA complexed with 18 (175 nM for SA and 25 nM for 18) by Trp-FRET. Tryptophan residues were excited at 295 nM, and FRET was measured at 460 nm. Values were corrected to account for fluorescence quenching upon binding as described in Experimental Section. [SA] was based on monomeric protein. Half-maximal inhibitory concentrations (IC50) were calculated using a log(inhibitor) vs response model (GraphPad Prism 6.0), and IC50 values were converted to Ki values. (C) Evaluation of direct binding of 20 to SA using ITC. Compound 20 was titrated into [SA] in PBS (pH 7.4), and thermodynamic parameters and Kd values were calculated using the Origin software. [SA in sample cell] = 20 μM and [20 in syringe] = 250 μM.
