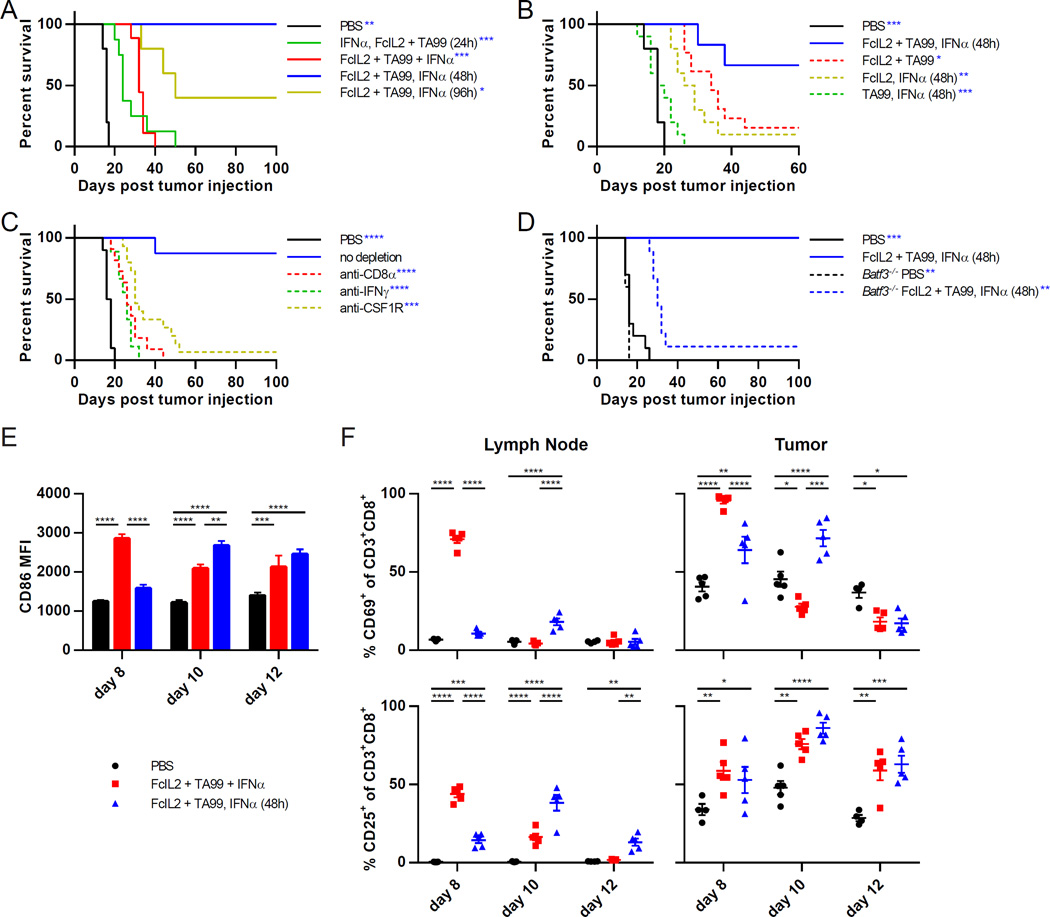
Figure 1. Relative Timing of Combination Immunotherapy Component Administration Determines Synergistic Antitumor Efficacy and Requires Specific Elements of Innate and Adaptive Immunity.
(A) Survival curves for mice injected s.c. with 106 B16F10 melanoma cells, then treated on days 6 and 12 with PBS or FcIL2 + TA99. Mice given FcIL2 + TA99 also received IFNα at indicated time points relative to FcIL2 + TA99 treatment. n = 5–9 per group.
(B) Survival curves for mice treated as described in (A), or with one of the three therapeutic components omitted. n = 5–13 per group.
(C) Survival curves for mice treated as described in (A). Mice given immunotherapy were also injected with the indicated depleting or neutralizing antibodies. n = 8–15 per group.
(D) Survival curves for wild-type or Batf3−/− mice treated as described in (A). n = 5–10 per group.
(E) MFI levels of CD86 expression by draining lymph node CD8α+ DCs (CD3−CD11chiPDCA-1−CD8α+) from immunotherapy-treated mice bearing established s.c. B16F10 tumors. n = 4–5 per group.
(F) Percentages of draining lymph node or intratumoral CD8+ T cells expressing CD69 or CD25. Cells were isolated from immunotherapy-treated mice bearing established s.c. B16F10 tumors. n = 4–5 per group.
Data represent mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 between the indicated pairs or versus the corresponding color group in the legend. See also Figures S1 and S2.