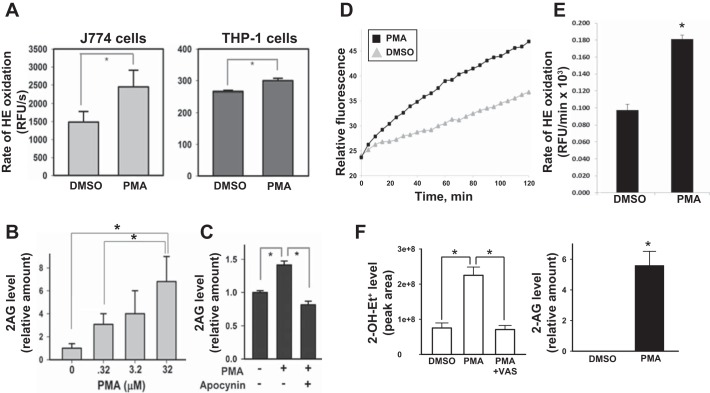
Fig. 4.
PMA-activated macrophages produce elevated amounts of superoxide and 2-AG. A: murine J774A.1 macrophages (left) and human THP-1 macrophages (right) were treated with either DMSO or PMA (0.32 μM) for 30 min and then loaded with the oxyradical probe HE (20 μM). The resulting fluorescence signal was monitored over 90 min at 37°C. PMA-treated J774A.1 macrophages and THP-1 macrophages exhibited a 65% and 13% increase in HE oxidation rates, respectively, compared with vehicle-treated control. RFU, relative fluorescence units. B: murine J774A.1 macrophages treated with the indicated amounts of PMA for 10 min exhibited a concentration-dependent increase in 2-AG production, suggesting that 2-AG biosynthesis is correlated with increased oxidative stress. C: pretreatment of J774A.1 macrophages with the Nox inhibitor apocynin abrogated PMA-stimulated production of 2-AG. D: human HL-60 cells were differentiated into macrophages (PMA, 100 nM, 72 h) and then treated with either PMA (3.2 μM) or DMSO, followed by the addition of HE (20 μM). The rate of HE oxidation was monitored in a fluorescent plate reader for 120 min. E: quantitative analysis of data shown in D is provided as a bar graph. F, left: levels of the superoxide-specific product 2-OH-Et+ were determined in HL-60 macrophages treated with either DMSO, PMA (3.2 μM), or PMA (3.2 μM) plus VAS (Nox inhibitor, 10 μM) for 30 min. The culture medium was removed, and cells overlaid with fresh medium containing 20 μM HE, followed by an additional incubation period of 20 min. Right: 2-AG levels were determined in HL-60 macrophages treated with either DMSO or PMA (3.2 μM, 30 min). Values are means ± SD (n = 3). *P < 0.05, Student's t-test when two groups are compared, and one-way ANOVA (Student-Newman-Keuls method) when more than two groups are compared.