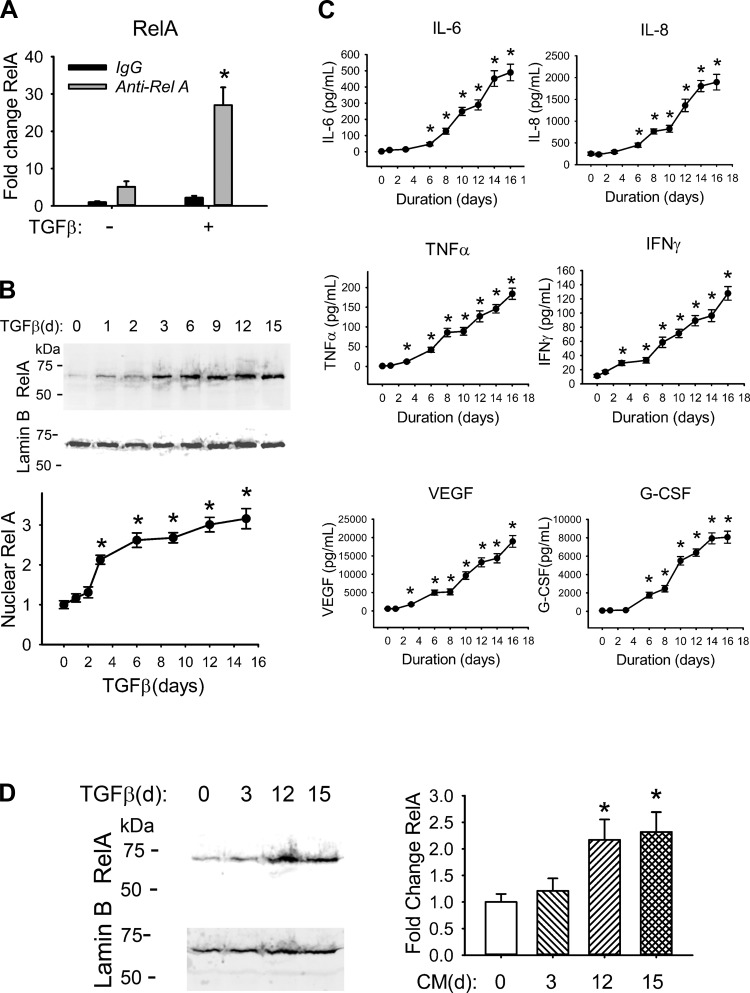
Fig. 1.
TGF-β activates the NF-κB signaling pathway. A: TGF-β increases total RelA abundance. hSAECs were incubated with TGF-β (10 ng/ml) for 15 days. Whole cell extracts of hSAECs were isolated and immunoprecipitated with primary anti-RelA Ab. RelA abundance was quantified by SID-SRM-MS. Shown is fold change abundance normalized to the input protein concentration. *P = 0.002 compared with mock treatment. B, top: TGF-β induces RelA nuclear translocation. hSAECs were incubated with a time series of TGF-β (10 ng/ml) for up to 15 days. One hundred fifty micrograms of nuclear extracts was processed for Western blot using anti-RelA Ab (top). Lamin B was detected as a loading control (bottom). Bottom: quantification of nuclear RelA. Shown is the fold change in protein abundance normalized to Lamin B. *P < 0.01 compared with mock treatment. C: TGF-β induces secretion of NF-κB-dependent cytokines/chemokines. hSAECs were incubated with a time series of TGF-β (10 ng/ml) up to 16 days. The conditioned medium was collected for cytokine determination by multiplex ELISA. Shown are the concentrations of IL-6, IL-8, TNF-α, IFN-γ, VEGF, and G-CSF. *P < 0.05 compared with control epithelial cells. D: conditioned medium from TGF-β-treated cells induces RelA nuclear translocation in naive hSAECs. Naive hSAECs were incubated for 1 h with 3-, 12-, or 15-day conditioned medium from TGF-β-treated hSAECs. Left: Western blot of 150 μg of nuclear extracts using anti-RelA Ab (top); the blot was probed with Lamin B as the loading control (bottom). Right: quantification of nuclear RelA. Shown is the fold change in protein abundance normalized to Lamin B. *P < 0.01 compared with mock treatment. All data shown are means ± SD from 3 independent experiments.