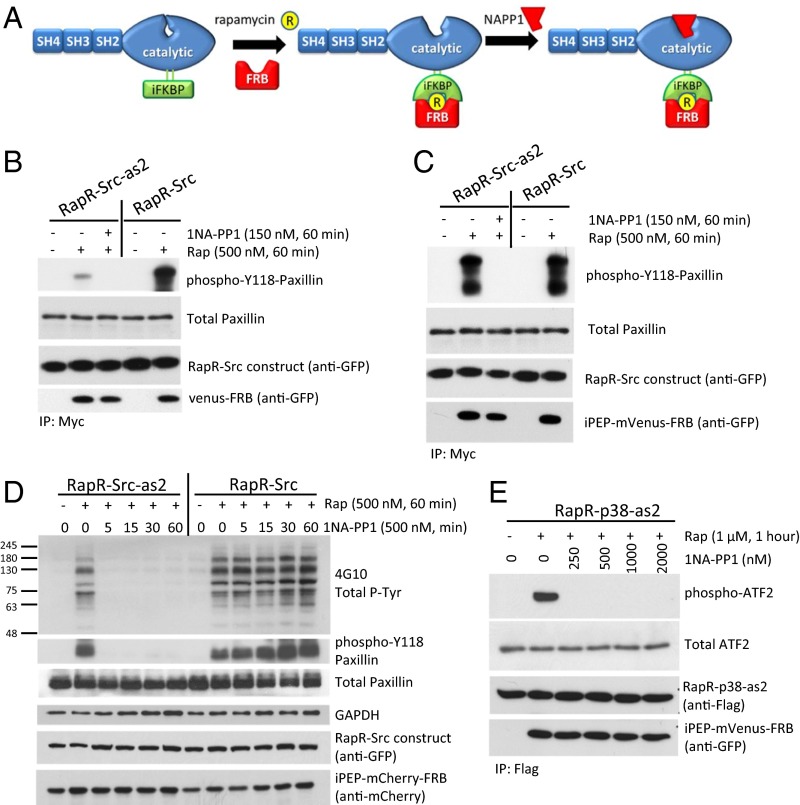
Fig. 1.
Transient activation of engineered RapR-as2 kinases. (A) Schematic of RapR-Src-as2 regulation. (B and C) In vitro kinase assay of immunoprecipitated Src constructs. We cotransfected LinXE cells with either (B) mVenus-FRB or (C) iPEP-mVenus-FRB and indicated Src constructs bearing both a cerulean tag (recognized by GFP antibody) and a myc tag. Cells were treated with rapamycin (Rap, 500 nM) or ethanol (solvent) for 1 h and immunoprecipitated. Kinase assays were carried out in the presence of equivalent volumes of either DMSO (solvent) or 1NA-PP1 (150 nM). The purified N-terminal fragment of paxillin was used as a substrate. (D) Regulation of Src activity in living cells. LinXE cells were cotransfected with iPEP-mCherry-FRB and either RapR-Src-as2-cerulean-myc or RapR-Src-cerulean-myc. Transfected cells were treated with rapamycin (500 nM) or ethanol (solvent) for 1 h and subsequently with 1NA-PP1 (500 nM) for the designated amount of time. Cell lysates were analyzed for total tyrosine phosphorylation and endogenous paxillin phosphorylation. (E) LinXE cells transfected with Flag-RapR-p38-as2and iPEP-venus-FRB were activated with rapamycin (1 μM) for 1 h. Five minutes before collection cells were exposed to UV for 30 s. Flag-RapR-p38-as2 was immunoprecipitated and analyzed by an in vitro kinase assay using ATF-2 as a substrate. 1NA-PP1 or DMSO (solvent) was added at the indicated dose to the kinase reactions. (B–E) Data are representative of at least three independent experiments.