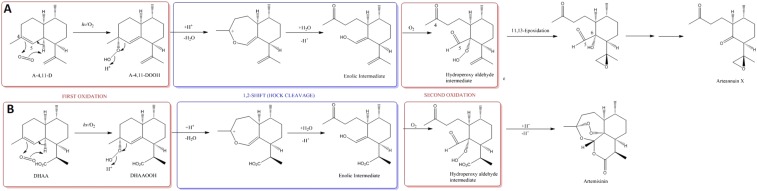
Fig. S6.
Theoretical scheme for the synthesis of arteannuin X from A-4,11-D (A) and comparison with the synthesis of artemisinin from DHAA (B). We propose that the first stages in the synthesis of arteannuin X proceed by the same mechanism that has been established for artemisinin (17) to effect oxidative cleavage at the C-4/C-5 position (A). Thus, A-4,11-DOOH, formed by an “ene-type” reaction of singlet oxygen with the 4,5-double bond in A-4,11-D, undergoes Hock cleavage to an enolic intermediate, which can then participate in a second oxidation to yield a hydroperoxy-aldehyde, similar to that produced from DHAOOH in the biogenesis of artemisinin (B). At this point in our proposed scheme, the synthetic routes to artemisinin and arteannuin X diverge. For artemisinin synthesis, the hydroperoxy-aldehyde intermediate from DHAA incorporates a carboxylic acid group at the 12 position which effectively “locks in place” the 3 new heterocyclic rings that are formed when the hydroperoxy-aldehyde “zips up” with the 4-keto and 5-aldehyde groups (B). For arteannuin X synthesis the hydroperoxy-aldehyde from A-4,11-D undergoes an alternative reaction, in which the terminal oxygen of the hydroperoxide is transferred to the 11,13-double bond, thereby producing the epoxide group found in arteannuin X (A). The final stages of the biogenesis of arteannuin X must then involve a second oxidative carbon–carbon bond cleavage reaction at C-5/C-6, in which C-5 is lost to form arteannuin X (A).