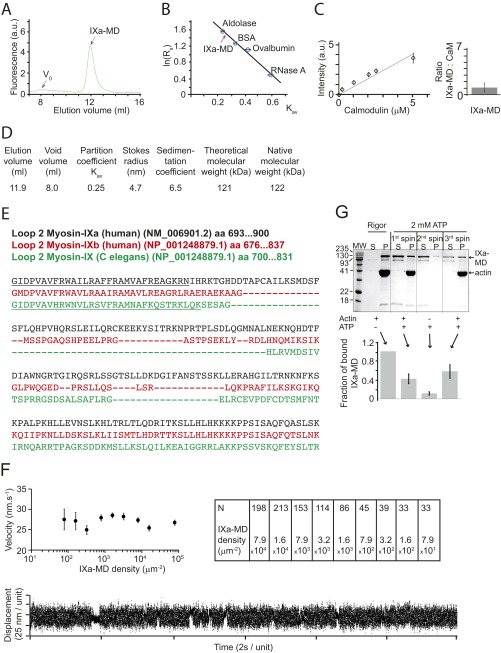
Fig. S1.
The IXa-MD binds calmodulin and binds to actin in the presence and absence of ATP. (A) Superdex-200 gel filtration showed that IXa-MD expressed in the presence of calmodulin eluted at 11.9 mL. (B) The Stokes radius of IXa-MD was determined from calibration curves using standard proteins of known Stokes radii as described (Stokes radii Rs; partition coefficient Kav) (40, 48). All experiments were performed at least three times. (C) Densitometry calibration using increasing calmodulin concentrations and determination of the molar ratio of IXa-MD to calmodulin in the purified complex in EGTA (Fig. 1B). (D) Using gel filtration and sucrose-density gradients, the myosin’s hydrodynamic properties were characterized as described (40, 48). The 122-kDa native molecular mass determined for IXa-MD was consistent with the theoretical value for monomeric IXa-MD. (E) Sequence analysis of loop 2 in IXa-MD indicated a 1-8-14 calmodulin-binding motif (underlined), as had been reported for other myosin-IX isoforms (C. elegans) (15). (F) Actin-gliding velocity at increasing densities of IXa-MD immobilized via a biotin–streptavidin linker on the surface of the experimental chamber (2 mM ATP; 22 °C). The table denotes the number of filaments analyzed in each experimental condition. In a single-molecule mechanical experiment using optical tweezers, IXa-MD produced single, intermittent interactions with actin, consistent with a nonprocessive motor. (G) Two forms of IXa-MD with different actin affinities interconverted. The SDS/PAGE gels and IXa-MD pellet (P)-band intensities were obtained by mixing 1.5 μM IXa-MD with 10 μM F-actin in the absence (rigor) or presence of 2 mM ATP, followed by a 20-min centrifugation at 435000 × g at 4 °C (first spin). In rigor, virtually all (and in the presence of ATP, 42 ± 10%) of IXa-MD pelleted with F-actin. From the IXa-MD remaining in the supernatant (S), 11 ± 4% pelleted following a spin performed without F-actin (second spin); 58 ± 14% of the IXa-MD remaining in S after the second spin pelleted with F-actin following a third spin. The fraction of bound IXa-MD is shown in percentage of the total IXa-MD (sum of S- and P-band intensities).