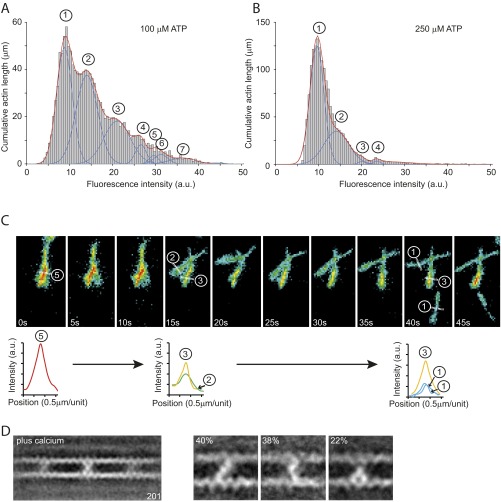
Fig. S2.
IXa-MD induced actin-bundle formation in the presence of ATP. The formation of actin bundles by IXa-MD at different nucleotide concentrations was analyzed using TIRF microscopy. Using an automated analysis procedure, the total number of pixels at different fluorescence intensities was determined and used to calculate the total length of actin bundles comprising two to seven actin filaments. (A) Distribution of fluorescence intensities across 100 fields of view (60 × 60 μm2) in the presence of 100 μM ATP. Up to seven filaments in a bundle could be resolved. (B) At 250 μM ATP, the number of filaments in the bundles was reduced, indicating that the formation of the bundles was fully reversible and dependent upon the concentrations of actin, IXa-MD, and ATP. (C) Disassembly of an actin bundle consisting of five filaments into smaller bundles and individual filaments over a period of 45 s in the presence of 250 μM ATP. The analysis of fluorescence intensity indicated that the formation of the bundle consisting of five filaments (0 s) was fully reversible. The bundle disassembled first into two bundles of two and three filaments (after 15 s) before single filaments detached from the bundle and reattached to the surface (40 s). (D) Ensemble average of 201 images in the presence of calcium (pCa 4.0). The cross-links can be divided into a diagonal-, bent-, and inchworm-type class.