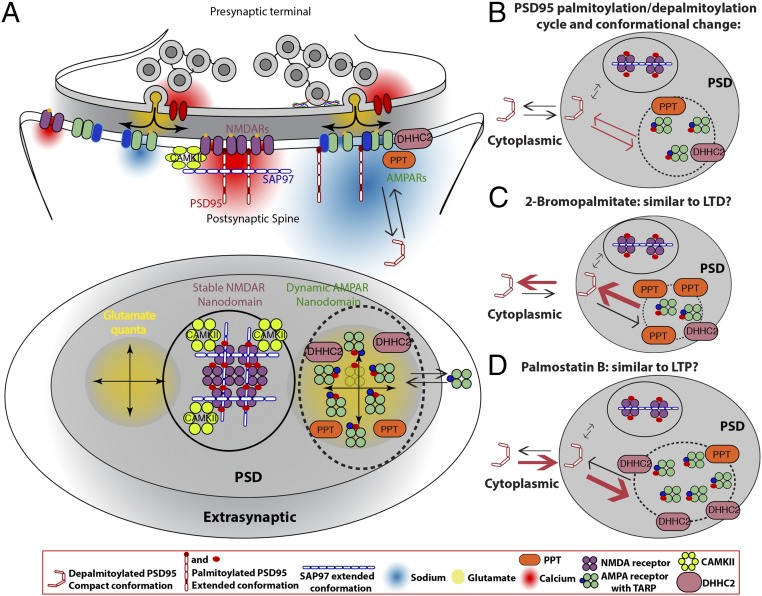
Fig. 5.
Model of PSD95 and SAP97 scaffolding separate AMPAR and NMDAR nanodomains and how the PSD95 palmitoylation cycle regulates AMPAR dynamics in PSDs. Based on our results and the work of others, we propose that AMPARs and NMDARs in PSDs are differentially scaffolded into separate nanodomains (NMDAR nanodomain, circle; AMPAR nanodomain, dotted circle). In the NMDAR nanodomains, stronger PSD95–SAP97 interactions stabilize NMDARs and prevent access of palmitoylation/depalmitoylation enzymes, DHHC2 and PPT. The more dynamic AMPAR nanodomain results from weaker interactions between PSD95 and TARP-containing AMPARs, and allows access of palmitoylation/depalmitoylation enzymes. (A) Lateral (Top) and en face (Bottom) schematic views of the presynaptic domain synapsing on the PSD) of a spine head. In the model, we propose functional rationales for why AMPARs and NMDARs cluster in separate nanodomains in PSDs. First, separate nanodomains increase receptor packing density, and thereby increase the sodium current density for AMPARs (red cloud) and calcium current density for NMDARs (yellow). Second, separate nanodomains allow strategic positioning of the nanodomains. As shown, positioning of the AMPAR nanodomain with respect to the presynaptic vesicle release site will regulate the size of the AMPAR response, whereas positioning of the NMDAR nanodomain with respect to downstream calcium response proteins will regulate intracellular calcium signaling at the PSD. Finally, separate domains allow other proteins to segregate in either AMPAR or NMDAR nanodomains. An example is shown in B–D for the palmitoylation of PSD95. (B–D) Details of how the palmitoylation cycle changes PSD95 conformation and its association with AMPARs and NMDARs. (B) PSD95 in the compact conformation attaches to the PSD and is stabilized there in the extended conformation when palmitoylated. In this conformation, PSD95 associates mainly with AMPAR subunits containing TARP subunits, such as Stargazin, by entering AMPAR nanodomains or, occasionally, with GluN2B NMDAR subunits in NMDAR nanodomains. The subunits dissociate from PSD95 when depalmitoylation reverses PSD95 conformation, changing back to the compact conformation. In this way, the PSD95 palmitoylation/depalmitoylation cycle regulates the number of AMPAR slots in AMPAR nanodomains. (C) We propose that the number of AMPAR slots, and thus the levels of AMPARs and PSD95, are decreased by decreasing PSD95 palmitoylation by DHHC2 blockade with 2-BP incubation. (D) Number of AMPAR slots, as well as the levels of AMPARs and PSD95, is increased by increasing PSD95 palmitoylation by PPT blockade with palmostatin B incubation.