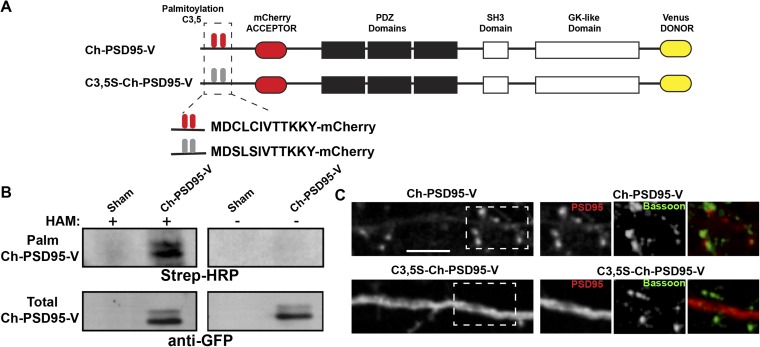
Fig. S1.
Further characterization of PSD95 and mutated PSD95 FRET sensors. (A) Schematic of PSD95 (Ch-PSD95-V) and mutated PSD95 (C3,5S-Ch-PSD95-V) FRET sensor constructs as in Fig. 1A. (B) Ch-PSD95-V is palmitoylated. Ch-PSD95-V was expressed in HEK293 cells and assayed for palmitoylation by the acyl-biotin exchange (ABE) assay. (Top) ABE assay comparing sham-transected HEK293 cells (sham) and cells transfected with Ch-PSD95-V. Specific labeling was only observed with the hydroxylamine (HAM) treatment step, a step required in the ABE method to assay for palmitoylation. (Bottom) Total cellular expression of Ch-PSD95-V was assayed by Western blots. (C) Distribution of Ch-PSD95-V and C3,5S-Ch-PSD95-V in dendrites. (Left) Cultured hippocampal neurons were transfected with Ch-PSD95-V (Top) or C3,5S-Ch-PSD95-V (Bottom). Displayed are images of mCherry fluorescent signal in dendrites for each construct. Ch-PSD95-V concentrated in spines, consistent with wild-type PSD95 distribution. C3,5S-Ch-PSD95-V had a more diffuse distribution consistent with disrupted targeting and accumulation at spines. (Scale bar: 5 μm.) [Right; Insets of dashed boxes (Left)] Distribution of Ch-PSD95-V and C3,5S-Ch-PSD95-V at synapses. Neurons were fixed and immunolabeled with anti-Bassoon antibody to identify presynaptic sites. Ch-PSD95-V, but not C3,5S-Ch-PSD95-V, colocalized with anti-Bassoon staining.