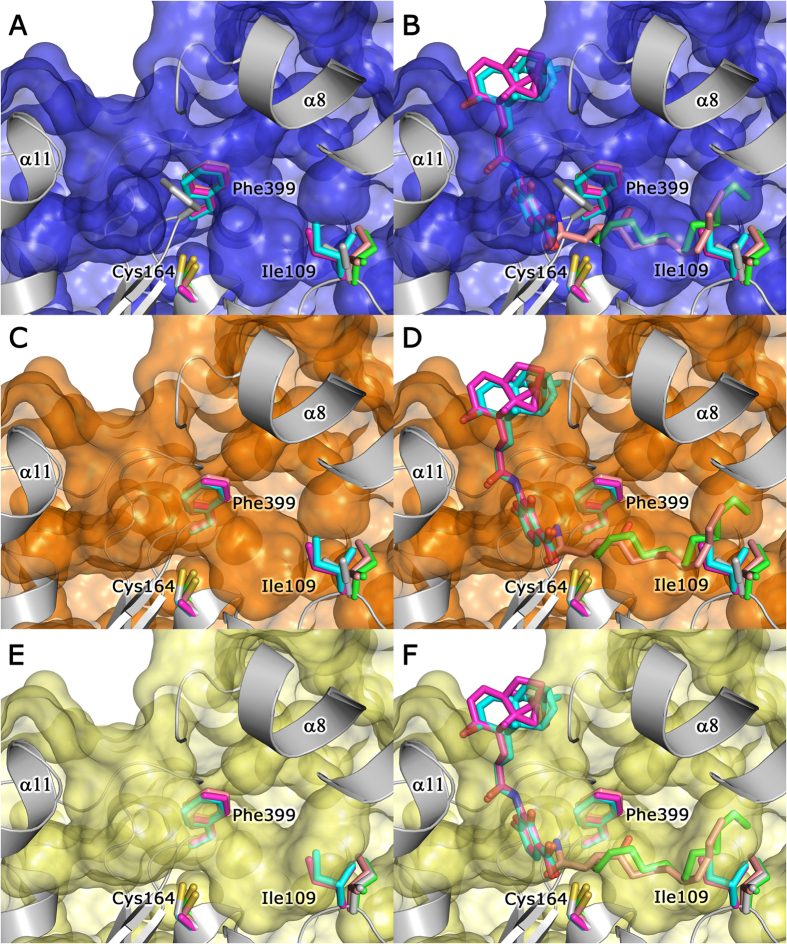
Figure 4. The LmFabF substrate binding pocket appears partially closed, and must undergo conformational changes in order to accommodate binding of substrates and inhibitors.
Superposition of the structures of E. coli FabF (EcFabF) in complex with cerulenin (PDB: 1B3N, pink), platencin (PDB: 3HO2, magenta), platensimycin (PDB: 3HNZ, cyan), and dodecanoic acid (PDB: 2GFY, green) shows that Phe399 and Ile109 of LmFabF (silver) partially close the substrate binding pocket (shown as a surface model) (A), clashing with cerulenin and dodecanoic acid (B). The binding pocket appears partially opened upon rotation of Phe399 (silver sidechain) to a conformation almost identical to that observed in the structures of EcFabF, overlapping with the phenylalanine sidechain of EcFabF (C). However, LmFabF is still unable to fully accommodate cerulenin and dodecanoic acid (D), with the additional rotation of Ile109 (silver sidechain) required to accommodate the entire acyl chain of these molecules (E,F). Note: platencin and platensimycin structures contain a Cys164Ala mutation.