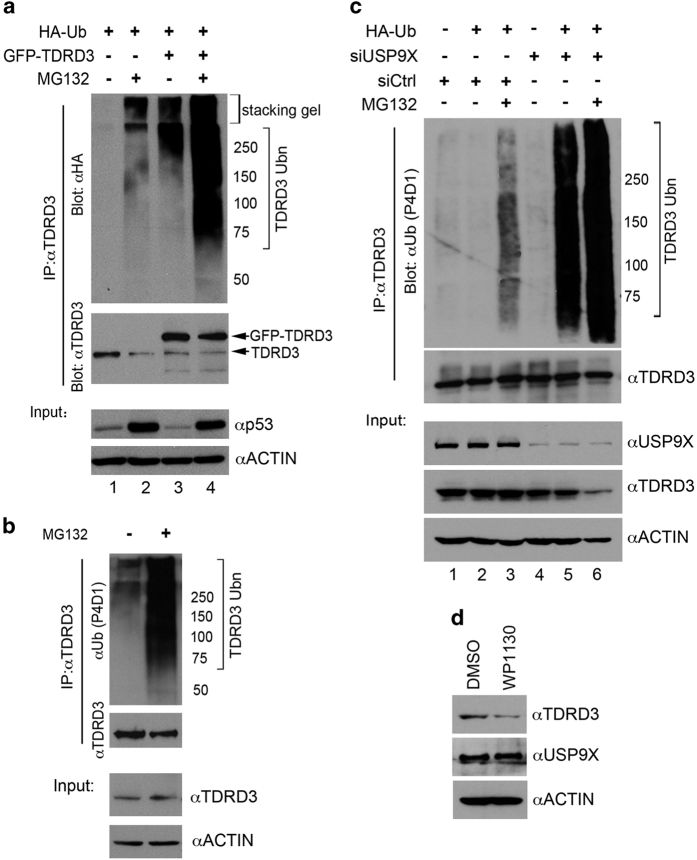
Figure 4.
USP9X protects TDRD3 from ubiquitination. (a) TDRD3 is ubiquitinated in cells. HeLa cells were transiently transfected with HA-ubiquitin and GFP-TDRD3 (as indicated). After 24 h of transfection, the cells were either treated with DMSO or 10 μm of MG132 for an additional 16 h. TDRD3 in vivo ubiquitination was detected by IP with anti-TDRD3. The eluted protein samples were detected with anti-HA and anti-TDRD3. The input samples were detected with anti-p53 and anti-ACTIN to monitor the efficiency of proteasome inhibition by MG132. (b) HeLa cells were treated with either DMSO or MG132 for 16 h and the total cell lysates were IPed with anti-TDRD3. The eluted protein samples were detected with anti-ubiquitin (P4D1) and anti-TDRD3. The input samples were detected with anti-TDRD3 and anti-ACTIN. (c) Loss of USP9X promotes TDRD3 ubiquitination in the cells. HeLa cells were transfected with either control or USP9X-specific siRNA and either treated with DMSO or MG132 for 16 h. The total cell lysates were IPed with anti-TDRD3 and the eluted protein samples were detected with anti-ubiquitin (P4D1) and anti-TDRD3. The input samples were detected with anti-USP9X, anti-TDRD3 and anti-ACTIN. (d) Inhibition of USP9X de-ubiquitinase (DUB) activity destabilizes TDRD3. HeLa cells were treated with 5 μm of WP1130 for 24 h. The expression levels of TDRD3 and USP9X were detected with anti-TDRD3 and anti-USP9X. Anti-ACTIN was used as a loading control.