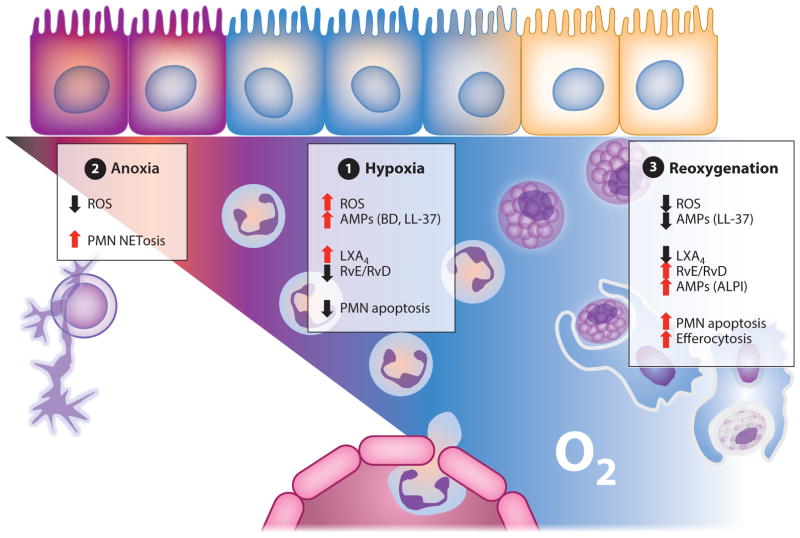
Figure 4.
O2-dependent regulation of AMPs; PMN fate in chronic inflammation and during resolution. (❶) Hypoxia: PMN induce localized hypoxia via NADPH oxidase activity, resulting in bacterial death by ROS generation. Hypoxia prolongs PMN life span, thereby preventing apoptosis. HIF-dependent AMPs are released and aid in antimicrobial activity. HIF-dependent glycolytic and proinflammatory genes (e.g., COX-2) are transcribed. RvE/RvD require the oxygenase activity of COX-2 and are synthesized. Activated PMN convert arachidonic acid to LXA4. (❷) Anoxia: In the absence of infection clearance, PMN accumulate and localized O2 depletion prevents NADPH oxidase activity. Under such conditions, PMN undergo NETosis or necrosis. (❶) Reoxygenation:With pathogenic bacterial clearance, PMN undergo apoptosis. RvE/RvD enhance clearance of apoptotic PMN through efferocytosis and induce intestinal ALPI expression on epithelia to restore mucosal homeostasis. Abbreviations: ALPI, alkaline phosphatase; AMP, antimicrobial peptide; HIF, hypoxia-inducible factor; PMN, polymorphonuclear leukocytes; ROS, reactive oxygen species; RvE/RvD, resolvins.