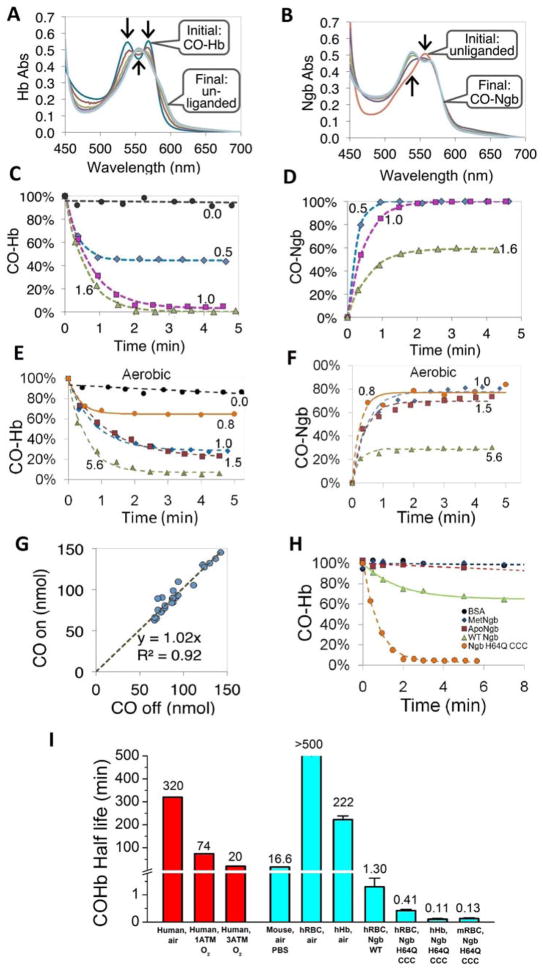
Fig. 3. CO transfer from RBC-encapsulated hemoglobin to Ngb-H64Q-CCC.
(A – B) Absorbance spectra of 40 μM Hb and 40 μM Ngb-H64Q-CCC, respectively, after mixing CO-Hb with deoxy-Ngb-H64Q-CCC at 37 °C in presence of 10 mM sodium dithionite. Arrows indicate the direction of absorbance changes. Initial spectra were recorded before mixing. (C – D) Time course of CO-Hb (C) and CO--Ngb-H64Q-CCC (D) concentrations after mixing at 37 °C, in the presence of 3 – 10 mM sodium dithionite. Numbers indicate the ratio of Ngb-H64Q-CCC to Hb. The time courses for equimolar amounts of Hb and Ngb were derived from the spectra shown in (A) and (B) respectively. (E – F). Time course of CO-Hb (E) and CO--Ngb-H64Q-CCC (F) under aerobic conditions at 37 °C. (G) The calculated moles of CO dissociated from Hb (x-axis) plotted against the calculated moles of CO bound to Ngb-H64Q-CCC, based on data in C – F, Fig. 2 and replicate experiments (n = 29). (H) Kinetic changes of RBC-encapsulated CO-Hb when mixed with BSA, met-Ngb-H64Q-CCC, apo-Ngb-H64Q-CCC, wild type Ngb and deoxy Ngb-H64Q-CCC, Protein:Hb ratios used were 2 for BSA and 1 for met-Ngb, ApoNgb, WtNgb and deoxy Ngb-H64Q-CCC. (I) Half-lives of CO dissociation from RBC-encapsulated or free Hb in mice and human. Human in vivo values were obtained from the literature (1, 34, 36). Other values were measured in this work. Error bars show SEM. The experiments were repeated at least three times.