Abstract
Verve, an oral nicotine delivery product (ONDP), was introduced by Nu Mark (Altria Client Group, Richmond VA) for smokers to use in places where smoking is prohibited. This study assessed the effect of this ONDP on plasma nicotine levels, heart rate, product satisfaction, and ability to suppress smoking urge and cigarette cravings. Thirteen daily cigarette smokers [8 men and 5 women; average age 33.4 years] attended two laboratory sessions, one occurred after overnight tobacco abstinence. Plasma samples were collected before and after ONDP use and measured for nicotine. In non-abstinent smokers, mean plasma nicotine levels increased from 18.3 to 21.0 ng/mL. In abstinent smokers, average nicotine levels increased from 3.1 to 4.5 ng/mL. After overnight tobacco abstinence, ONDP use significantly (p < 0.01) increased heart rate from 69 beats per minute (bpm) to 75 bpm; while urge to smoke decreased significantly (p < 0.01) from a score of 8.6 to 4.9. Participants indicated moderate product satisfaction that was not changed by tobacco abstinence. Analysis of unused ONDP revealed total nicotine levels of 1.68 ± 0.09 mg/disc. Spent ONDP discs were also analyzed to determine % nicotine liberated during chewing; results were 80% in the non-abstinent and 82% in the abstinent conditions (ns). Our study results indicate that ONDP use can increase plasma nicotine levels and heart rate and reduce cigarette cravings in abstinent smokers.
Keywords: Oral nicotine delivery product (ONDP), Verve, Nicotine pharmacokinetics, Tobacco
1. Introduction
In recent years the tobacco industry has introduced a number of new tobacco products marketed to smokers as cigarette alternatives in situations where tobacco smoking is prohibited (e.g. workplace, airplanes, public places). The use of these products may involve oral administration or inhalation of a substance aerosol (e.g., Electronic Nicotine Delivery systems (ENDs)) rather than tobacco combustion (Palazzolo, 2013). Some examples of these products include snus, a low nitrosamine product that originated in Sweden (Stepanov et al., 2012), and dissolvable products that consist of compressed tobacco with added flavorings that fully dissolve in the mouth, (e.g. Camel Sticks, Strips and Orbs) (Rainey et al., 2011; Connolly et al., 2010) or dissolves from a solid support, such as a toothpick (Marlboro Tobacco Sticks; Skoal Tobacco Sticks). Although some smokers quit tobacco products altogether using novel oral use products, many smokers may use alternate products concurrently with their typical smoked products; thus preventing or delaying tobacco cessation and resulting in continued exposure to both nicotine and other toxicants (Palazzolo, 2013).
In 2012, an ONDP called Verve was introduced and continues to be sold in test markets in Virginia by NuMark, a company associated with Altria Client Group (Philip Morris). This spit-free product is a nondissolving, solid polymer disc containing non-tobacco cellulose fibers, flavorings and reportedly 1.5 mg of tobacco-derived nicotine that is released to users when the disc is chewed (Liu et al., 2013). This ONDP product has similar characteristics as medicinal nicotine products such as nicotine gum sold for smoking cessation.
The ONDP utilizes a nicotine-embedded matrix to provide buccal absorption similar to formulations of nicotine polacrilex (gum). Oral tobacco products may have public health consequences in nonsmokers and smokers who use these products (Tomar, 2007). Use of moist snuff has been shown to initiate cigarette smoking (Tomar and Giovino, 1998) and the use of oral tobacco products may postpone cessation attempts (Tomar, 2003; Connolly et al., 1986; Agaku et al., 2013). The present study assessed pharmacologic effects (nicotine delivery) and cardiovascular parameters (heart rate and blood pressure), and performed qualitative assessment of perceptions, health risks, and ability to suppress smoking urge and cigarette cravings in daily cigarette smokers. Furthermore, ONDP discs were characterized according to several physical (disc weight, % moisture) and chemical parameters (pH, total nicotine and calculated nonprotonated nicotine). Nicotine content and pH are particularly important measures because they directly affect the quantity and speed of nicotine absorption (Fant et al., 1999; Pickworth et al., 2014; Benowitz et al., 1987, 1988).
The Food and Drug Administration (FDA) is currently considering scientific data relevant to its regulatory authority over tobacco products, particularly ones such as ONDP that are not specifically mentioned in the Family Smoking Prevention and Tobacco Control Act (FSPTCA) (Public Law 111-31, 2009). These types of products are marketed as alternatives to cigarettes; however, some nicotine-dependent users may continue smoking while using these products in settings where smoking is prohibited or unacceptable.
2. Materials and methods
2.1. Participants
Thirteen daily cigarette smokers (8 males; 7 African Americans, 6 Whites) participated in this study. The average age of the participants was 33.4 (range: 21–52). The participants smoked an average of 16.9 ± 3.5 cigarettes/day (CPD) with a range of 10–20 CPD. The smokers in this study had smoked regularly for an average of 15 ± 9.4 years (range: 3–31 years). None of the participants had used this ONDP prior to the study and none were current users of oral tobacco products. Their most commonly used cigarette brands were: Newport (8), Marlboro (2), Camel (1), L&M (1), and Maverick (1). All participants smoked filtered cigarettes (10 smoked menthol and 3 smoked non-menthol). Participants had moderate to strong levels of nicotine dependence as indicated by the average Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) of 5.8 ± 2.1 (range 1–8); a score of 5 or greater is generally indicative of nicotine dependence. Data were collected between December 2012 and February 2013 at Battelle's Human Exposure Assessment Laboratory (Baltimore, MD).
2.2. Oral nicotine delivery product (ONDP)
The ONDP discs used in the present study were purchased from a convenience store in the Fredericksburg, VA area in October of 2012. According to product marketing materials, the product contains approximately 1.5 mg of tobacco-derived nicotine. The ONDP is sold in a plastic tube containing 16 discs each. The mint-flavored ONDP discs are flat, Reuleaux triangular-shaped with a dimension of approximately 3 mm (thickness) × 15 mm × 12 mm and weigh 500 mg.
2.3. Product measurements of total and non-protonated nicotine
Nicotine concentrations in unchewed and chewed discs were measured by gas chromatography–mass spectrometry (GC/MS) in selected ion monitoring (SIM) mode as described elsewhere (Stanfill et al., 2009) with slight modifications to extraction conditions, which are described below. In order to validate the extraction efficiency, nicotine from unchewed ONDP discs was extracted under standard and modified conditions. It was determined that samples were most thoroughly extracted after sonication for 3 h at 60 °C; these modified parameters were used to analyze all of the ONDP disc samples. The pH measurements, required for calculating non-protonated nicotine, were made using a Sirius Vinotrate pH robot (Sirius Analytical Ltd., East Sussex, UK), which adds a 5-mL aliquot of distilled, deionized water to each sample and makes temperature-corrected pH measurements. Product pH was the averaged value of pH measurements at 5, 15, 30 and 60 min. Calculations for the percentage of nicotine as non-protonated nicotine were taken from the Federal Register (Federal Register, 1999). Nicotine values (total and non-protonated) are expressed as mg/disc. The ONDP discs were also analyzed for minor alkaloids (nornicotine, mysomine, anatabine, and anabasine) to assess whether the nicotine is synthetic or purified from tobacco. Minor alkaloid analysis was performed using GC/MS/MS according to a previously published method (Lisko et al., 2013). The method limits of detection (LODs) for the minor alkaloids are 0.08, 0.04, 0.12 and 0.12 µg/g for nornicotine, myosmine, anabasine and anatabine, respectively.
2.4. Methods and procedure
Research volunteers were screened for eligibility and interest in the study during a telephone interview. Potential participants were invited to the laboratory and assessed for general health and the accessibility of an arm vein for blood draws, and also screened for concomitant medications. Participants signed a consent form that had been reviewed and approved by the Battelle Institutional Review Board and were paid a total of $150 for the two visits. A Research Determination Form was completed for this activity and it was determined that the role of the Centers for Disease Control (CDC) did not constitute engagement in human subject research.
Participants made two visits to the clinic: the first when they were allowed to use tobacco before the session and the second after overnight tobacco abstinence where they were prohibited from using tobacco or nicotine products. During clinical visits, participants were seated in a comfortable chair. Eating, drinking and smoking were prohibited during the session. Exhaled carbon monoxide (CO) was collected at the beginning of the session. After a 5 minute rest, baseline heart rate (HR) and blood pressure (BP) readings were recorded and the participant completed the Questionnaire on Smoking Urges short form (QSU) (Cox et al., 2001). A butterfly needle was inserted in an arm vein and a blood sample was collected. The ONDP was given to the participants who chewed it ad lib for 15 min as directed by the labeled instructions of the manufacturer. At 5 and 10 minute marks, HR was recorded. At the end of the 15 min, the participant removed the spent ONDP and placed it into a tube that was refrigerated. HR, BP and exhaled CO were recorded and another blood sample was collected. The participant answered Visual Analog Scale questionnaires, Product Satisfaction Scale and Risk Perception Questions. The procedure at the second visit was identical except that the participant must have been tobacco abstinent for at least 12 h as verified by an exhaled CO level below 13 ppm. Following their collection, samples (unchewed and chewed discs) were shipped to CDC on dry ice for analyses.
2.5. Dependent measures
2.5.1. Physiologic measures
HR and BP were recorded before, during (5, 10 min) and at the conclusion of ONDP use using a finger pulse oximeter unit and an automated monitor (DRE Waveline Plus Vital Signs Monitor [DRE, Inc., Louisville, KY]). In order to verify overnight abstinence from tobacco and other combustible products, exhaled CO levels were determined before and after ONDP use using a Breath CO monitor (Vitalograph, Lenexa, KS). Plasma samples were taken from a 7 mL blood draw before and within 5min after the 15 minute-ONDP use period. Blood plasma was generated by centrifugation and stored frozen until analysis by Labstat International, LLC (Kitchener, Ontario). The nicotine limit of quantification (LOQ) of LabStat's TME-00001 method was 4.1 ng/mL; the limit of detection (LOD) was 1.2 ng/mL. For sample values between LOD and LOQ, the level was estimated from extrapolation from the standard curve [LabStat, personal communication].
2.6. Subjective measures
Before and after ONDP administration, participants completed the brief form (10 questions) of the QSU (Cox et al., 2001). The QSU yields a Total Score (overall craving) and Factor 1 (craving defined by anticipation of positive effects of smoking) and Factor 2 (craving derived from anticipated relief of tobacco withdrawal discomfort). Product satisfaction was assessed by four Visual Analog Scale questions that measured “strength”, “liking”, “head rush”, and “alert”. The participant indicated their endorsement by placing a mark on a line anchored by the phrases “not at all” and “extremely”. The response was the percentage of the entire line (0 to 100). A 16-item questionnaire derived from items from the Cigarette Evaluation Scale (CES) (Westman et al., 1992) and the Duke Sensory Questionnaire (DSQ) (Behm and Rose, 1994) were used to assess product liking and effects. All questions were answered on a 1 to 7 scale (1 = not at all; 7 = extremely). Perceptions of the health risk of the ONDP were assessed using five questions: “I think it would be safe to use”; “I would buy it”; “compared to your usual brand of cigarettes ONDP contains more, less about the same nicotine (or don't know)”; “more, less or equally safe or don't know”; and “would the ONDP make it easier to quit smoking”. The Minnesota Withdrawal Scale (Hughes et al., 1991) is a multiple item standard test for the severity of signs and symptoms associated with tobacco withdrawal.
3. Results
3.1. ONDP characteristics
To characterize the ONDP, the Tobacco and Volatiles Branch, Division of Laboratory Sciences at the Centers for Disease Control (CDC) and Prevention measured the ONDP discs for several physical (disc weight, % moisture) and chemical parameters (pH, total nicotine and calculated non-protonated nicotine) (Table 1). All measured values are presented on a per disc basis. The average weight of the ONDP discs was 493 mg and each disc had an average moisture content of 8.4%. The measured value of total nicotine (1.68 mg) is 11% higher than the stated value of 1.5 mg on the product website (NuMark website, 2014). The aim of the analysis was to measure the maximum extractable nicotine.
Table 1.
Measured values for disc weight, size dimensions, percent moisture, pH, % non-protonated nicotine, and total nicotine concentrations in unused ONDP discs.
| Parameters for unused ONDP discs | Average values (±SD) |
|---|---|
| Disc weight (mg)a | 493.0 ± 12.8 |
| Percent moisturea | 8.40 ± 0.05 |
| pHb | 7.47 ± 0.09 |
| Nicotine (mg/disc, stated on packaging)c | 1.50 |
| Measured total nicotine (mg/disc)b | 1.68 ± 0.09 |
| % of nicotine as non-protonated nicotine (at pH 7.47) | 22.1% |
| Non-protonated nicotine (mg/disc at pH 7.47)b | 0.37 |
ONDP = oral nicotine delivery product.
n = 5.
n = 4.
n = 3.
Using the Henderson–Hasselbalch equation, the percentage of nicotine in the non-protonated form for the unchewed discs was calculated to be 22.0% (0.37 mg/disc). In unchewed discs from freshly opened packs, the average aqueous pH was 7.47. Chewed discs from participants who did not finish the protocol were available for pH analysis. The pH values from four partially chewed samples ranged from pH 7.31 to 7.92; at those pH values, the percent of nicotine in the nonprotonated form ranged from 16.2 to 44.2%.
3.2. ONDP nicotine release
Spent discs were analyzed from participants in the non-abstinent (Visit 1) and abstinent (Visit 2) conditions. As shown in Table 2, total nicotine liberated (removed by chewing) from the chewed discs ranged from 0.83 to 1.68 mg/disc, which corresponds to 49.2–100% total nicotine liberated upon use.
Table 2.
Visit 1 and Visit 2: ONDP discs chewed by non-abstinent/abstinent smokers. Amount of nicotine retained (mg) and % released from ONDP discs. The calculated values are based on a total nicotine value of 1.68 mg/disc.
| Non-abstinenta | Abstinent | |||
|---|---|---|---|---|
| Participant number |
Nicotine (mg/disc) |
% total nicotine |
Nicotine (mg/disc) |
% total nicotine |
| Post-chewing | Released | Post-chewing | Released | |
| 1 | 0.21 | 87% | 0.24 | 86% |
| 2 | 0.04 | 98% | 0.08 | 95% |
| 9 | 0.36 | 79% | 0.14 | 92% |
| 10 | 0.31 | 82% | 0.25 | 85% |
| 13 | 0.86 | 49% | 0.75 | 55% |
| 17 | 0.29 | 83% | 0.23 | 86% |
| 18 | 0.60 | 65% | 0.84 | 50% |
| 21 | 0.58 | 66% | 0.30 | 82% |
| 24 | n.d. | 100% | 0.03 | 98% |
| 28 | n.d. | 100% | n.d. | 100% |
| 32 | 0.70 | 59% | 0.68 | 59% |
| 33 | n.d. | 100% | n.d. | 100% |
| Mean ± SD | 0.34 ± 0.29 | 80 ± 18% | 0.30 ± 0.29 | 82 ± 18% |
ONDP = oral nicotine delivery product.
n.d. — not detectable.
For calculation purposes 0.5 × LOD = 0.025 mg/disc was used.
Participants were tested on one day without smoking restrictions (non-abstinent) and on another day after overnight tobacco abstinence (abstinent).
Table 2 shows the amount of nicotine retained in the ONDP discs after chewing. Nine participants had results above the limit of detection; the data from one participant was incomplete and not included in the analysis. In both the abstinent and non-abstinent conditions, a similar amount of nicotine (range of 0 to 0.85 mg/disc of nicotine) remained in the disc after chewing. Across the nine participants the amount of nicotine released from the disc ranged from 49% to 100% (0.83–1.68 mg).
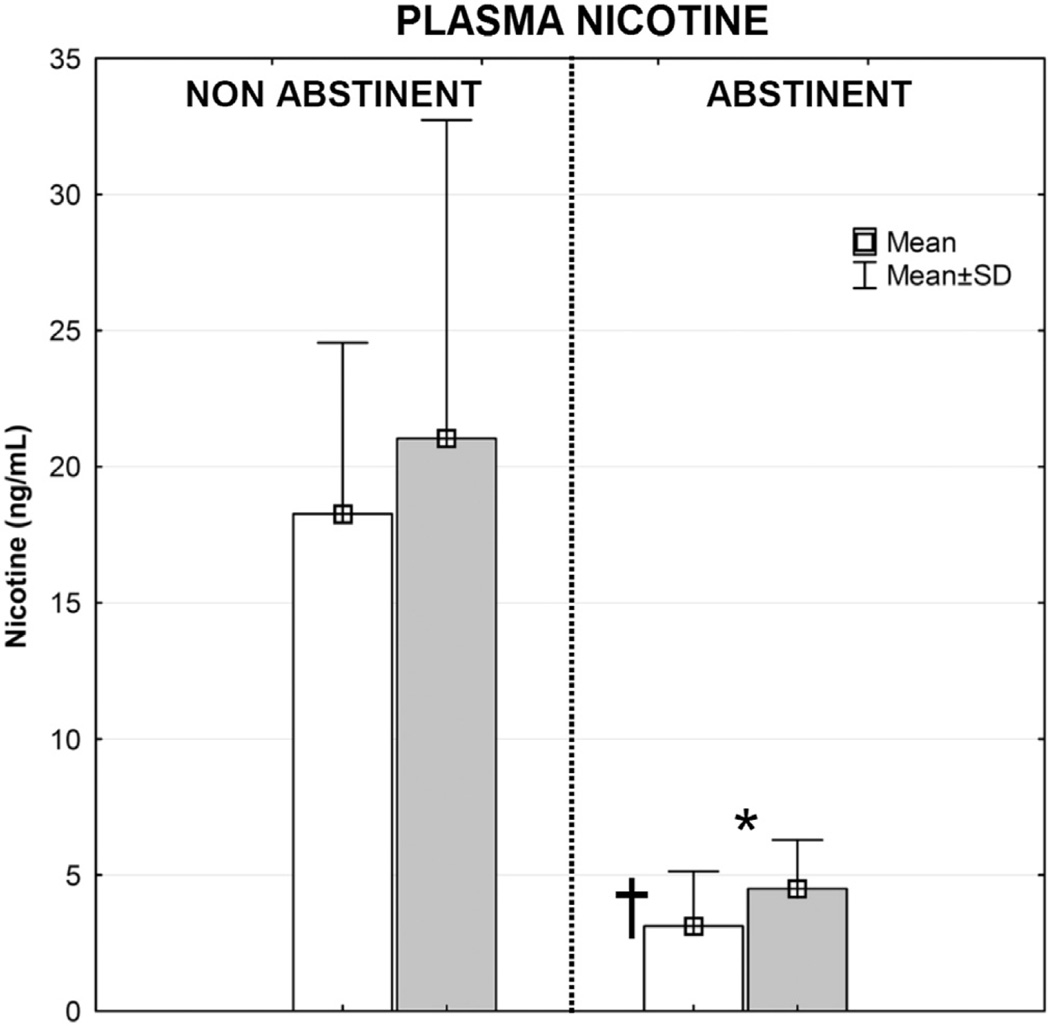
Exhaled CO values and plasma nicotine levels confirm that participants complied with the overnight tobacco restrictions. Average exhaled CO values on the non-abstinent day (26.9 ppm) and abstinent day (6.6 ppm) were significantly different (p < 0.05). As shown in Fig. 1, decreases in plasma nicotine levels were observed on the abstinent day. After overnight tobacco abstinence, plasma levels of nicotine decreased from an average of 18.3 ± 6.3 ng/mL to 3.1 ± 2.0 ng/mL (p < 0.01). The ONDP delivered nicotine in quantities sufficient to raise plasma nicotine levels by 2.7 and 1.4 ng/mL in the non-abstinent and abstinent conditions, respectively. There was a statistically significant (p < 0.05) decrease in the mean heart rate before ONPD use on the abstinent day (69 ± 5 bpm) compared to the heart rate before ONDP on the non-abstinent day (75 ± 8 bpm) (Fig. 2). After ONDP use on the abstinent day, heart rate rapidly and significantly increased to levels similar to the resting heart rate observed on the non-abstinent day.
Fig. 1.
ONDP plasma nicotine delivery in non-abstinent and abstinent conditions. White bar—before chewing; gray bar—after chewing; *—difference before/after chewing in abstinent condition (p = .0566); † — difference before chewing in non-abstinent/abstinent condition (p < .01).
Fig. 2.
Heart rate during ONDP chewing (non-abstinent and abstinent conditions). Mean HR values with standard error are presented; PRE — base (resting) heart rate measured before chewing; 5 min, 10 min, and 15 min — HR measured in 5th and 10th minutes of chewing and immediately after 15 min of chewing; * — statistically significant difference between non-abstinent and abstinent conditions (p < 0.05); † — statistically significant difference before/after chewing in abstinent condition.
Overnight tobacco abstinence significantly increased scores on the Minnesota Nicotine Withdrawal Scale from an average of 8.8 ± 5.5 to 13.0 ± 9.6 (p < 0.05). Overnight tobacco abstinence led to significant increases in cigarette craving. Average scores for Factors 1 and 2 and Total Score of the QSU significantly increased at baseline of the abstinent compared to the non-abstinent day. After use of the ONDP on the abstinent day there was a significant reduction in tobacco craving on all metrics: 5.5 (initial) vs 3.7 (post-use) for Factor 1, (p < 0.05); 3.1 vs. 1.3 (Factor 2, p < 0.01), and 8.6 vs 4.9 (Total Score, p < 0.01). However, the ONDP-induced reductions in QSU scores were not significant on the non-abstinent day.
Responses to a series of subjective questionnaires indicated moderate liking and acceptability of the product; the subjective assessments did not change during the abstinent condition. For example, on the 5-point scales of whether they would purchase the ONDP, the average response was between “not certain” and “maybe”; likewise, the product was less acceptable if it were the same price as cigarettes. Compared to their usual cigarette participants indicated that ONDP delivers the same amount of nicotine and that they were about as “safe” as cigarettes. There were no significant differences in the ONDP product evaluation on the non-abstinent and abstinent days. On a scale of 0 to 100, the average value for ‘liking’ this products was 42.1; the perceived strength rating averaged 67.2. The product was perceived as ‘strong’ (Participants 28, 21, 2, and 9) by those subjects with the highest nicotine extraction and higher nicotine boost (0.8–3.6 ng/mL). It was liked most by two people (Participants 9 and 21) who had disc extractions of 87 and 93% and had increases of plasma nicotine levels of 0.8 and 3.6 ng/mL, respectively.
4. Discussion
Tobacco lozenges, dissolvable tobacco products and ENDs have been introduced and marketed as products that can be used by smokers in situations where smoking is prohibited. The ONDP examined in the present report is unique because it is marketed by a tobacco company as a substitute for cigarettes; whereas, other oral nicotine-only products are sold by pharmaceutical companies for the purpose of smoking cessation. A public health concern is that nicotine delivery products such as ONDPs and ENDs that relieve craving in abstinent situations or in situations where combustible smoking is not allowed may reduce the motivations of smokers to quit (Palazzolo, 2013).
In the present study, we found that the ONDP delivered small amounts of nicotine. The increase in plasma nicotine after 15 min of use averaged 2 ng/mL and was similar in both tobacco-abstinent and non-abstinent participants. These results indicate a similar extraction efficiency regardless of the cigarette craving. The increase in plasma nicotine was similar in magnitude to that seen with other tobacco products 1.5-mg Ariva and 4-mgStonewall (Kotlyar et al., 2007), prototype ONDP products (Liu et al., 2013), and the 2-mg nicotine gum (Lunell and Lunell, 2005; Benowitz et al., 1987), but smaller than plasma nicotine levels after a lozenge containing 4-mg of nicotine (Kotlyar et al., 2007). The increase in plasma nicotine after the ONDP is much less than the 11 ng/mL increase typically observed after cigarette smoking (Williams et al., 2010) or after conventional moist snuff use where plasma levels increased between 4.2 and 19.5 ng/mL (Fant et al., 1999; Kotlyar et al., 2007; Pickworth et al., 2014).
Although the increase in plasma nicotine levels after ONDP use was quite small compared to cigarette smoking, the ONDP caused significant increases in heart rate in tobacco-abstinent participants. The increase in heart rate of about 6 beats per minute (bpm) was similar in magnitude to that seen after nicotine gum use but less than the 10 to 12 bpm increase following cigarette use in nicotine-deprived smokers (Benowitz et al., 1988). Also, despite the small increases in plasma nicotine, a decrease in cigarette craving occurred. The marketing of the ONDP is directed at the momentary relief of tobacco cravings and smoking urges. The data from the present study support reduced cigarette craving as measured by the decrease in QSU scores. Nicotine polacrilex, (Nicorette™ gum), also temporarily decreases tobacco cravings and these effects are most evident when tobacco craving is increased after periods of tobacco deprivation (Houtsmuller et al., 2002; Kotlyar et al., 2007).
Participants reported ‘low’ to ‘moderate’ scores on scales of product liking and willingness to purchase the ONDP. However, ‘liking’ for a new product is typically quite low among established tobacco users that have self-selected a product from the vast array of oral and combustible tobacco products in the market (Chen et al., 2010; Borgerding et al., 2012). ‘Liking’ scores for nicotine polacrilex are also typically quite low (Pickworth et al., 1986; Houtsmuller et al., 2002; Nemeth-Coslett et al., 1988). The limited and rather slow nicotine absorption from the ONDP and the low to moderate ratings on product ‘liking’ suggest an overall low to moderate risk of abuse from this ONDP — similar to that of other oral nicotine delivery products.
It is interesting to note that participants had the perception that ONDP delivered about the same amount of nicotine as their brand of cigarette even though the plasma nicotine boost was only 20% of the boost associated with cigarette smoking. Moreover, participants expressed no difference in this response whether asked on the abstinent or nonabstinent day. Ad lib use of the nicotine polacrilex (gum 2 mg) typically releases about 50% of its nicotine content (Pickworth et al., 1986) and increases plasma levels of nicotine by about 3 ng/mL (Lunell and Lunell, 2005; Kotlyar et al., 2007) whereas, the participants in the present study were able to extract between 50 and 100% of the nicotine from their ONDP, suggesting that the formulation of this ONDP provides for a more efficient release of nicotine.
The discs were analyzed in triplicate forminor alkaloids and found to contain nornicotine, myosmine, anabasine and anatabine. Minor alkaloids were quantified using a recently developed GC/MS/MS analytical method (Lisko et al., 2013). Concentrations of minor alkaloids were found to be higher than the LOD for each analyte but below the lowest calibrator. The presence of nornicotine, mysomine, anatabine, and anabasine suggests that the nicotine present in this product is derived from a highly purified tobacco extract rather than a synthetic source. This is an important finding because the FDA is restricted by the FSPTCA to the regulation of tobacco products or products made from tobacco (Public Law 111-31, 2009). The chemical analysis further support the notion that this ONDP is made from tobacco derived nicotine and may be subject to FDA regulation.
Limitations of the present study include using only a single dose of the ONDP, a relatively small number of participants and lack of a placebo condition. However, the results indicate that the ONDP delivers doses of nicotine capable of suppressing craving, and increasing heart rate in tobacco-abstinent smokers. It is difficult to accurately predict the amount of nicotine that was absorbed because physiological factors such as oral pH, saliva volume, buffering potential of saliva, and saliva swallowing vary among participants. In future studies, the measurement of salivary pH before and after ONDP use would be helpful in the interpretation of data.
5. Conclusions
Like the current dramatic increase in use of other non-traditional nicotine delivery products (e.g., ENDs) (Palazzolo, 2013), dissolvable tobacco (Rainey et al., 2011), and ONDP-like products pose important questions for tobacco control policy. In addition to delivery of nicotine, unique characteristics of the ONDP may also have public health implications — their use is easily concealed and the product is spit-less. These characteristics may increase the appeal of ONDP to people including pregnant women or youth, who may wish to conceal their nicotine use. Furthermore, because the ONDP provides for nicotine delivery in places where smoking is not allowed, they may subvert the effects of indoor smoking regulations that tend to increase smoking cessation (Hopkins et al., 2010). The ONDP delivered small levels of nicotine in this study, suggesting that these products will not fully satisfy cravings and may lead to ongoing or concurrent use of ONDP with other tobacco products. The ONDP could provide nicotine without use of combusted tobacco products; but low amounts of nicotine delivered to the user suggest that they could be a nicotine ‘bridge’ to the next cigarette. Therefore, use of ONDP or similar products may prevent people who might otherwise quit from achieving complete cessation.
Acknowledgments
We gratefully acknowledge the assistance of Dr. Lacy Fabian and Natalia Ceaicovscaia for the proof reading and editorial assistance. Funding for this study was provided by Battelle Internal resources.
Biographies

Bartosz Koszowski, Battelle Memorial Institute

Stephen Stanfill, Centers for Disease Control and Prevention

Joseph G Lisko, Centers for Disease Control and Prevention

Zach Rosenbery, Battelle Memorial Institute
Footnotes
CDC Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
Disclosures
The authors declare no conflict of interests.
References
- Agaku IT, Ayo-Yusuf OA, Vardavas CI, Alpert HR, Connolly GN. Use of conventional and novel smokeless tobacco products among US adolescents. Pediatrics. 2013;132(3):e578–e586. doi: 10.1542/peds.2013-0843. http://dx.doi.org/10.1542/peds.2013-0843 (doi: peds.2013-0843 [pii], Retrieved from PM:23918889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm FM, Rose JE. Reducing craving for cigarettes while decreasing smoke intake using capsaicin-enhanced low-tar cigarettes. Exp. Clin. Psychopharmacol. 1994;2:143–153. [Google Scholar]
- Benowitz NL, Jacob P, III, Savanapridi C. Determinants of nicotine intake while chewing nicotine polacrilex gum. Clin. Pharmacol. Ther. 1987;41(4):467–473. doi: 10.1038/clpt.1987.58. (doi: 0009-9236(87)90136-6 [pii]. Retrieved from PM:3829583) [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P., III Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin. Pharmacol. Ther. 1988;44(1):23–28. doi: 10.1038/clpt.1988.107. (Retrieved from PM:3391001) [DOI] [PubMed] [Google Scholar]
- Borgerding MF, Bodnar JA, Curtin GM, Swauger JE. The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul. Toxicol. Pharmacol. 2012;64(3):367–387. doi: 10.1016/j.yrtph.2012.09.003. http://dx.doi.org/10.1016/j.yrtph.2012.09.003 (doi: S0273-2300(12)00182-1 [pii], Retrieved from PM:23000415) [DOI] [PubMed] [Google Scholar]
- Chen C, Isabelle LM, Pickworth WB, Pankow JF. Levels of mint and wintergreen flavorants: smokeless tobacco products vs. confectionery products. Food Chem. Toxicol. 2010;48(2):755–763. doi: 10.1016/j.fct.2009.12.015. http://dx.doi.org/10.1016/j.fct.2009.12.015 (doi: S0278-6915(09)00597-3 [pii], Retrieved from PM:20034536) [DOI] [PubMed] [Google Scholar]
- Connolly GN, Winn DM, Hecht SS, Henningfield JE, Walker B, Jr, Hoffmann D. The reemergence of smokeless tobacco. N. Engl. J. Med. 1986;314(16):1020–1027. doi: 10.1056/NEJM198604173141605. http://dx.doi.org/10.1056/NEJM198604173141605 (Retrieved from PM:3515184) [DOI] [PubMed] [Google Scholar]
- Connolly GN, Richter P, Aleguas A, Jr, Pechacek TF, Stanfill SB, Alpert HR. Unintentional child poisonings through ingestion of conventional and novel tobacco products. Pediatrics. 2010;125(5):896–899. doi: 10.1542/peds.2009-2835. http://dx.doi.org/10.1542/peds.2009-2835 (doi: peds.2009-2835 [pii], Retrieved from PM:20403932) [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. (Retrieved from PM:11260806) [DOI] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob. Control. 1999;8(4):387–392. doi: 10.1136/tc.8.4.387. (Retrieved from PM:10629244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Register. Annual Submission of the Quantity of Nicotine Contained in Smokeless Tobacco Products Manufactured, Imported, or Packaged in the United States (FR Doc. 99–7022) 1999:14085–14096. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. (Retrieved from PM:1932883) [DOI] [PubMed] [Google Scholar]
- Hopkins DP, Razi S, Leeks KD, Priya KG, Chattopadhyay SK, Soler RE. Smokefree policies to reduce tobacco use. A systematic review. Am. J. Prev. Med. 2010;38(2 Suppl):S275–S289. doi: 10.1016/j.amepre.2009.10.029. http://dx.doi.org/10.1016/j.amepre.2009.10.029 (S0749-3797(09)00751-X [pii], Retrieved from PM:20117612) [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol. Biochem. Behav. 2002;72(3):559–568. doi: 10.1016/s0091-3057(02)00723-2. (doi: S0091305702007232 [pii]. Retrieved from PM:12175452) [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: A replication and extension. Arch. Gen. Psychiatr. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, et al. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tob. Control. 2007;16(2):138–142. doi: 10.1136/tc.2006.018440. http://dx.doi.org/10.1136/tc.2006.018440 (doi: 16/2/138 [pii], Retrieved from PM:17400953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisko JG, Stanfill SB, Duncan BW, Watson CH. Application of GC–MS/MS for the analysis of tobacco alkaloids in cigarette filler and various tobacco species. Anal. Chem. 2013;85(6):3380–3384. doi: 10.1021/ac400077e. http://dx.doi.org/10.1021/ac400077e (Retrieved from PM:23394466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang Q, Rimmer L, Sarkar M. Characterization of plasma profile from a single use of two prototype oral tobacco products in adult smokers. Annual Meeting of the Society for Research on Nicotine and Tobacco; 2013; Boston, USA. 2013. [Google Scholar]
- Lunell E, Lunell M. Steady-state nicotine plasma levels following use of four different types of Swedish snus compared with 2-mg Nicorette chewing gum: a crossover study. Nicotine Tob. Res. 2005;7(3):397–403. doi: 10.1080/14622200500125468. http://dx.doi.org/10.1080/14622200500125468 (doi: J2312K3410272757 [pii], Retrieved from PM:16085507) [DOI] [PubMed] [Google Scholar]
- Nemeth-Coslett R, Benowitz NL, Robinson N, Henningfield JE. Nicotine gum: chew rate, subjective effects and plasma nicotine. Pharmacol. Biochem. Behav. 1988;29(4):747–751. doi: 10.1016/0091-3057(88)90197-9. (Retrieved from PM:3413200) [DOI] [PubMed] [Google Scholar]
- Palazzolo DL. Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review. Front. Public Health. 2013;1(56) doi: 10.3389/fpubh.2013.00056. http://dx.doi.org/10.3389/fpubh.2013.00056 (Retrieved from PM:24350225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Herning RI, Henningfield JE. Electroencephalographic effects of nicotine chewing gum in humans. Pharmacol. Biochem. Behav. 1986;25(4):879–882. doi: 10.1016/0091-3057(86)90401-6. (Retrieved from PM:3786346) [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Rosenberry ZR, Gold W, Koszowski B. Nicotine absorption from smokeless tobacco modified to adjust pH. J. Addict. Res. Ther. 2014;5(3) doi: 10.4172/2155-6105.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Law 111-31 § 3(1) 2009 [Google Scholar]
- Rainey CL, Conder PA, Goodpaster JV. Chemical characterization of dissolvable tobacco products promoted to reduce harm. J. Agric. Food Chem. 2011;59(6):2745–2751. doi: 10.1021/jf103295d. http://dx.doi.org/10.1021/jf103295d (Retrieved from PM: 21332188) [DOI] [PubMed] [Google Scholar]
- Stanfill SB, Jia LT, Ashley DJ, Watson CH. Rapid and chemically selective nicotine quantification in smokeless tobacco products using GC–MS. J. Chromatogr. Sci. 2009;47(10):902–909. doi: 10.1093/chromsci/47.10.902. (Retrieved from PM:19930803) [DOI] [PubMed] [Google Scholar]
- Stepanov I, Biener L, Knezevich A, Nyman AL, Bliss R, Jensen J, et al. Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: findings from Round 1 of the New Product Watch. Nicotine Tob. Res. 2012;14(3):274–281. doi: 10.1093/ntr/ntr209. http://dx.doi.org/10.1093/ntr/ntr209 (doi: ntr209 [pii], Retrieved from PM:22039075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar SL. Is use of smokeless tobacco a risk factor for cigarette smoking? The U.S. experience. Nicotine Tob. Res. 2003;5(4):561–569. doi: 10.1080/1462220031000118667. (doi: JN4WYKHFM8W793FV [pii]. Retrieved from PM:12959794) [DOI] [PubMed] [Google Scholar]
- Tomar S. Epidemiologic perspectives on smokeless tobacco marketing and population harm. Am J Prev Med. 2007;33(6 Suppl):S387–S397. doi: 10.1016/j.amepre.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Tomar SL, Giovino GA. Incidence and predictors of smokeless tobacco use among US youth. Am. J. Public Health. 1998;88(1):20–26. doi: 10.2105/ajph.88.1.20. (Retrieved from PM:9584028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Levin ED, Rose J. Smoking while wearing the nicotine patch: is smoking satisfying or harmful? Clin. Res. 1992;40:871A. [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Shen J, Foulds J, et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob. Res. 2010;12(8):855–859. doi: 10.1093/ntr/ntq102. http://dx.doi.org/10.1093/ntr/ntq102 (doi: ntq102 [pii], Retrieved from PM:20584771) [DOI] [PMC free article] [PubMed] [Google Scholar]