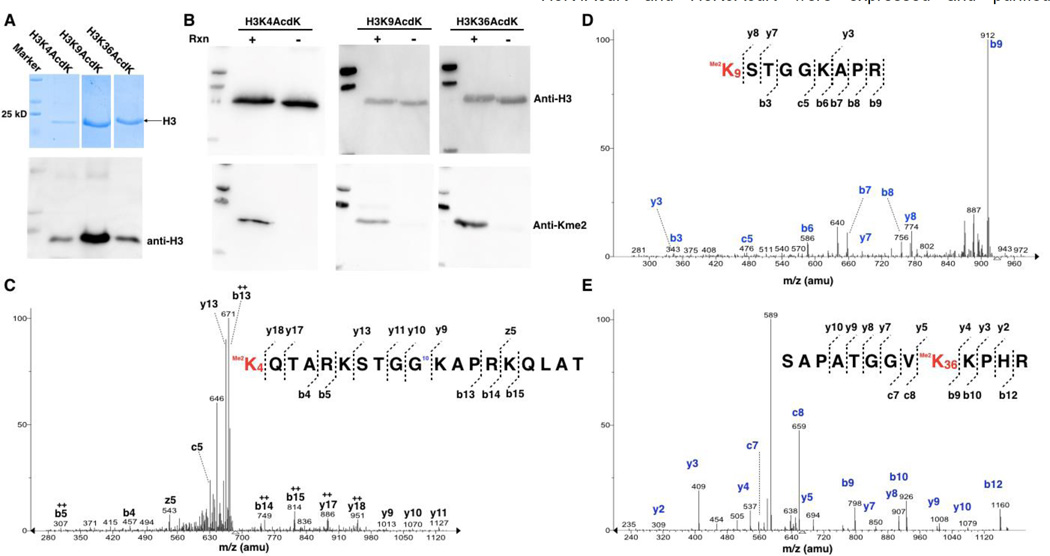
Figure 3. The synthesis of H3K4me2, H4K9me2, and H3K36me2.
(A) SDS-PAGE and western blot analyses of purified AcdK-containing H3 variants. Two plasmids pEVOL-AcdKRS and petDeut-1-H3 with a gene coding H3 with an amber mutation were used to co-transform E. coli BL21(DE3) cells. The transformed cells were grown in LB medium to OD 0.8. H3 expression was induced with the addition of 0.2% arabinose, 1 mM IPTG, and 2 mM AcdK. The expressed H3 variants were purified using Ni-NTA resins. (B) The Western blotting analysis of AcdK-containing H3 variants after their Staudinger reduction with TCEP and reductive amination with dimethylamine. Reaction conditions are ss same as shown in Figure 2D. (C–E) Tandem mass spectrometry analysis of Kme2-containing and trypsinized fragments of H3K4me2, H3K9me2, and H3K36me2.