Abstract
Objective:
Biebersteinia (Geraniaceae) has a history of use in traditional medicine in some countries including Iran. In the present study, cytotoxic and apoptogenic properties of hydro-ethanol extract of B. multifidi was investigated on human prostate cancer cell lines (PC3 and DU 145) and human embryonic kidney 293 (HEK293) cells.
Materials and Methods:
Cells were cultured in RPMI-1640 medium supplemented with 10% FBS at 37ºC in a humidified atmosphere of 95% air and 5% CO2. The root of the plant was macerated with EtOH 70%. Cytotoxic activity of ethanol extract of B. multifida was assessed using alamarBlue® assay after 48 hr of treatment. Apoptotic cells were stained with propidium iodide (PI) and detected by flow cytometry (sub-G1 peak).
Results:
B. multifidi had cytotoxic effect on malignant cells and normal HEK293 cells in a dose-dependent manner and significantly decreased the cell viability (IC50 values were between 199.2 and 302.9 µg/ml). B. multifida increased the sub-G1 peak in flow cytometry histogram of treated PC3 cells compared to control showing the induction of apoptosis and DNA fragmentation.
Conclusion:
Due to cytotoxic and apoptotic activity of B. multifida, the plant is suggested for further phytochemical analysis and mechanistic evaluation.
Key Words: Biebersteinia multifidi, Cytotoxicity, Apoptosis, Geraniaceae
Introduction
Medicinal plants have played a major role in cancer treatment and prevention. Many of natural-based therapeutics have clinical applications based on preliminary screening (Mousavi et al., 2015 ▶). Apoptosis is proposed as the major death pathway by chemotherapeutic agents and there are many natural chemotherapeutic agents which interfere with both extrinsic and intrinsic pathways of apoptosis (Safarzadeh et al., 2014 ▶).
Geraniaceae family has five genera in Iran and Biebersteinia is one of them. Biebersteinia has four species in the world and B. multifida DC. is the only species which is grown in Iran (Janighorban, 2009 ▶). The plant is found in many provinces of Iran and also is found in Caucasia, central Asia, Afghanistan, Iraq and Lebanon. B. multifida has yellow flowers and thick root and is of 20-70 cm height (Muellner, 2011 ▶).
In traditional medicine, the roots of B. multifida is used to relieve muscle pain which may be attributed to anti-inflammatory and analgesic properties of the plant (Farsam et al., 2000 ▶). Also, it is used for the treatment of children nocturia, phobia and anxiety (Monsef-Esfahani et al., 2013 ▶). It has been shown that B. multifida is effective in rehabilitating bone fractures and decreasing the severity of catatonia following treatment with anti-psychotic drugs (Khakpour and Hadipour, 2008 ▶). Previously published data shows the presence of polysaccharides, peptide, alkaloids like vasicinone, and flavonoids including 7-glucosides of apigenin, luteolin, and tricetin, as well as 7-rutinoside of apigenin and luteolin in this plant (Greenham et al., 2001 ▶; Omurkamzinova et al., 1991 ▶). Vasicinone has been shown to be responsible for some of the pharmacological effects of the plant such as antioxidant and antihemolytic activities (Monsef-Esfahani et al., 2013 ▶; Greenham et al., 2001 ▶).
There are no reports on the apoptotic activity of B. multifida and the aim of this study was to evaluate the cytotoxic and apoptotic activity of the plant on DU 145 and PC3 as two human prostate cancer cell lines.
Materials and Methods
Reagents and chemicals
AlamarBlue® (resazurin) was obtained from Sigma (Saint Louis, MO, USA). RPMI-1640 and fetal bovine serum (FBS) were bought from Gibco (Grand Island, NY, USA). Fluorescent probe propidium iodide (PI) was purchased from Sigma (Steinheim, Germany). All the solvents used for extraction were purchased from Caledon and Scharlau (Spain).
Plant Materials
The roots of B. multifida were collected from Asadli valley (1340 m height) in Asadli, a village 30 km far from Bojnurd, Nourth Khorasan province, northeast of Iran. The plant was identified by Mr. Imani (Research Center of Natural Products Safety and Medicinal Plants). Voucher specimen (No. npmp45) was deposited in herbarium of North Khorasan University of Medical Sciences.
The root of the plant (100 g) was dried under standard condition away from light, powdered by electrical grinder and kept in freezer. For preparing the extract of the root, the maceration method with ethanol 70% was used. The obtained filtrate was then dried at 30-40ºC. This yielded 10.2 g of dry extract (10.2%) which was dissolved in dimethylsulfoxide (DMSO) and then was subjected to cytotoxic and apoptosis assays. The concentration of DMSO in sample test was lower than 0.05% (Ahmadzadeh et al., 2014 ▶).
Cell cultures
The human prostate cancer cells (DU 145 and PC3) and human embryonic kidney 293 (HEK293) cells were maintained in RPMI-1640 medium supplemented with 10% FBS, penicillin 100 U/ml, and streptomycin 100 µg/ml at 37ºC in a humidified atmosphere of 95% air and 5% CO2. The stock solutions were prepared at 100 mg/ml in dimethylsulfoxide and kept at -20ºC.
For alamarBlue® assay, cells were seeded at 104 cells per well into 96-well culture plates. For assay of apoptosis, cells were seeded at 104 cell per well onto a 24-well plate. For each concentration and time course study, there was a control sample that remained untreated and received the equal volume of DMSO.
Cell viability
Resazurin, the active ingredient of alamarBlue® is an indicator which is reduced and converted to resorufin in live and healthy cells. This conversion changes the color of resazurin, a cell permeable redox indicator, from blue to the red and highly fluorescent compound named resorufin (O'Brien et al., 2000 ▶; Boozari et al., 2015 ▶).
Cells were treated with various concentrations of hydro-ethanol extract of B. multifida. After 44 hr incubation, alamarBlue® was added to each well and the absorbance was measured at 570 nm and 600 nm by a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA Winooski is a city in ChittendenWinooski is a city in Chittenden).
The IC50 values of B. multifida extracts were calculated using Graph Pad Software (Graph Pad prism 5 software). Cell viability of three independent experiments each in triplicate was presented as mean ± SD.
PI Staining
PI stained treated cells were evaluated by flow cytometry to analyze the apoptotic cells as represented by increased sub-G1 peak in the related histogram and compared to control (Nicoletti et al., 1991 ▶; Motaez et al., 2015 ▶). When stained with quantitative DNA-binding dye PI, DNA small fragments are lost in apoptotic cells incubated in a hypotonic phosphate-citrate buffer containing triton-X100 and will appear to the left of the G1 peak. Briefly, PC3 cells were treated with different concentrations (25, 50, 100 and 200 µg/ml) of hydro-ethanol extract of B. multifida for 48 hr. Floating and adherent cells were then harvested and incubated at 4°C for 4 hr in the dark with 400 μl of a hypotonic buffer (PI 50 μg/ml in 0.1% sodium citrate plus 0.1% Triton X-100) before flow cytometric analysis using a FACScan flow cytometer (Becton Dickinson). Here, 104 events were acquired with FACS.
Statistical analysis
One way analysis of variance (ANOVA) and Bonferroni’s post-hoc were used for data analysis. All results were expressed as mean ± SD and p values below 0.05 were considered statistically significant.
Results
Inhibition of cell viability
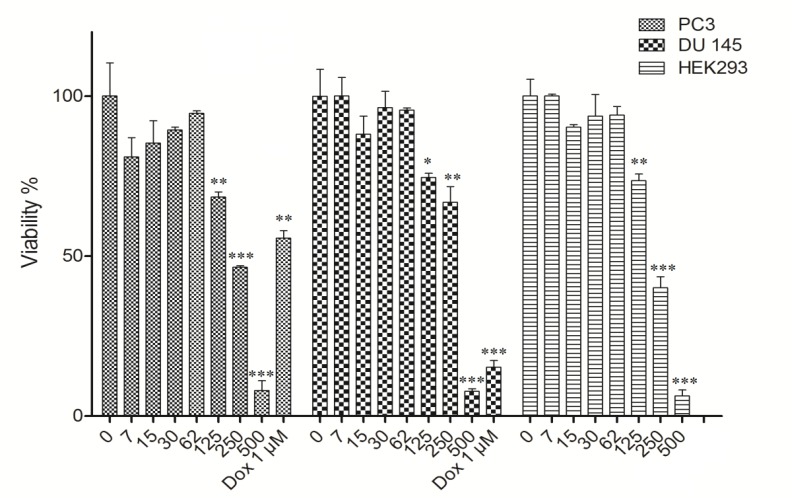
Inhibition of cell viability caused by hydro-ethanol extract of B. multifida was examined using alamarBlue® assay. The results showed that the hydro-ethanol extract of B. multifida decreased cell viability in a concentration-dependent manner (Figure 1) and significantly decreased the cell viability at the concentration of 125 µg/ml and higher (p< 0.05). The concentrations hydro-ethanol extract of B. multifida inducing 50% cell growth inhibition (IC50) in PC3, DU 145 and HEK293 cells for are presented in Table 1. Doxorubicin at the concentration of 1 μM was used as the positive control which decreased the cell viability of DU 145 and PC3 cells to 17±3.1% and 55±6.3%, respectively.
Figure 1.
A) Dose-dependent growth inhibition of PC3, DU 145 and HEK-293 cells by hydro-ethanol extract of B. multifida (0, 7, 15, 30, 60, 125, 250 and 500 µg/ml) after 48 hr. Viability was quantitated by alamarBlue® assay. The doses inducing IC50 against PC3, DU145 and HEK-293 cells by hydro-ethanol extract of B. multifida were 299.5, 302.9 and 199.2 µg/ml, respectively. Doxorubicin (1 µM) was used as the positive control. Results are mean±SD (n = 9). ∗p <0.05, ∗∗p <0.01 and ∗∗∗p <0.001 compared to control
Table 1.
IC50 values (µg/ml) for ethanol extract of B. multifida in PC3, DU 145 and HEK293 cell lines.
| Cell Line | IC 50 ( µg/ml) | IC 50 range |
|---|---|---|
| PC3 | 299.5 | 250.3-358.4 |
| DU 145 | 302.9 | 250.4-366.5 |
| HEK293 | 199.2 | 176.5-224.9 |
Apoptosis induction by hydro-ethanol extract of B. multifida in PC3 and DU 145 cells
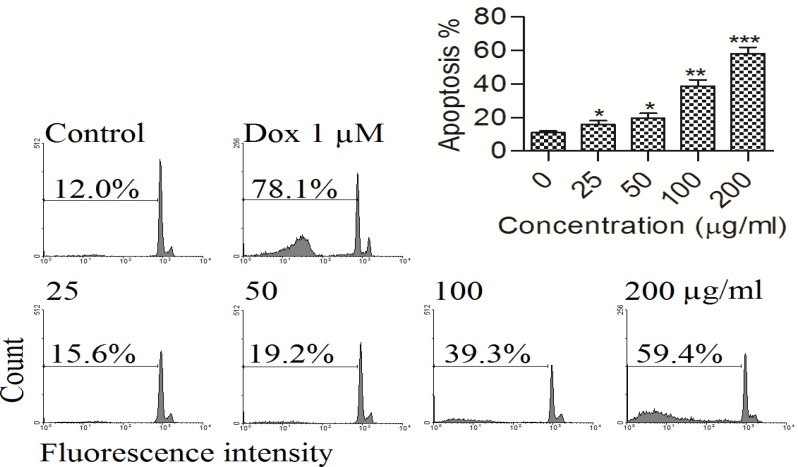
The PC3 cells treated with 25, 50, 100 and 200 µg/ml hydro-ethanol extract of B. multifida for 48 hr, induced a sub-G1 peak in flow cytometry histogram compared to untreated control cells (Figure 2) which shows the significant apoptosis induction by the extract (p<0.05 compared to control).
Figure 2.
A) Flow cytometry histograms of apoptosis assays by PI method in PC3 cells. Cells were treated with different concentration of hydro-ethanol extract of B. multifida (0, 25, 50, 100 and 200 µg/ml) for 48 hr. Sub-G1 peak as an indicative of apoptotic cells, was induced in hydro-ethanol extract of B. multifida-treated but not in control cells. Treated cells exhibited a sub-G1 peak in PC3 cells in a concentration-dependent manner that indicates the involvement of an apoptotic process in hydro-ethanol extract of B. multifida -induced cell death. Results are representative of three independent experiments. ∗p <0.05, ∗∗p <0.01 and ∗∗∗p <0.001 compared to control
Discussion
Plants have been served as valuable sources of phytochemicals with various biological effects. Plants and phytochemicals are widely used in treatment of diseases including cancer. Screening of plant and phytochemicals has led to identification of some of the famous groups of chemotherapeutics like taxanes, camptothecins, podophyllotoxins and vinca alkaloids (Fulda, 2010 ▶). In this study, we investigated the putative cytotoxic activity of hydro-ethanol extract of B. multifida on PC3, DU 145 and HEK293 cells. After searching for cytotoxic activity of the plant, the apoptotic activity was also explored on PC3 cells. At a range of 0-500 μg/ml, after 48 hr of treatment, the extract decreased the viability of cells in a dose-dependent manner in treated cells. The apoptotic activity was verified in PC3 cells at concentrations of 25, 50, 100 and 200 μg/ml. The subG1 peak in treated cells was increased in a dose-dependent manner which shows the involvement of apoptosis in cytotoxic activity of B. multifida.
Literature search shows that B. multifida has been investigated for different biological activities. Hashem Dabaghian et al. reported that the plant has anticancer potential as the ethanolic extract of B. multifida has the ability to prevent the reverted mutations in antimutagenicity test. They also showed the cytotoxic activity of B. multifida in human leukemia pre B-cells (Hashem Dabaghian et al., 2014 ▶). In similar evaluation of methanol extract of 15 Iranian medicinal plants including B. multifida, the plant showed cytotoxic activity on MCF-7, HepG2 and WEHI cells (Sahranavard et al., 2009 ▶). In this study, apoptosis-inducing activity of the plant has been showed for the first time.
When evaluated for antioxidant activity, the radical scavenging activities of the essential oil and methanol extract of fruits of B. multifida were superior to some other essential oils and extracts tested for this purpose. Additionally, B. multifida could effectively inhibit the oxidation of the linoleic acid comparable to butylated hydroxytoluene, curcumine and ascorbic acid (Amiri, 2009). It is reported that different levels of antioxidant and antihemolytic activities of B. multifida may be attributed, at least in part, to the presence of phenols and flavonoids in the extracts (Nabavi et al., 2010). Phytochemical studies showed that B. multifida consists of neutral polysaccharides named glucans A, B, and C (Arifkhodzhaev et al., 1985 ▶; Arifkhodzhaev and Rakhimov, 1993 ▶, 1994) and alkaloids (Kurbanov and Zharekeev, 1974 ▶). In this regard, biological activities reported for B. multifidi may be associated with the presence of phenols, flavonoids and alkaloid contents of the plant. This study reported the cytotoxic and apoptotic activity of B. multifida. The range of IC50 values for the plant was between 199.2 and 302.9 µg/ml hydro-ethanol extract. Although there are some reviews which suggested that active cytotoxic plants should have IC50s lower than 100 µg/ml on cancer cells (Taylor et al., 2014 ▶) but there are some active phytochemicals like artemisinin, artesunate and artenimol-R which have been purified from the plants with IC50s higher than the acceptable range (Efferth et al., 2011 ▶; Ericsson et al., 2014 ▶; Jansen et al., 2011 ▶). In a cytotoxic evaluation of Artemisia annua L., authors reported “The range of IC50 values for HeLa cancer cells was 54.1-275.5 μg/ml for dichloromethane extracts and 276.3-1540.8 μg/ml for methanol extracts” (Efferth et al., 2011 ▶).
Fifty two and fifty three compounds were identified in two independent studies from the essential oil of B. multifida after hydrodistillation and gas chromatography which mainly includes (E)-nerolidol, hexadecanoic acid, phytol, and 6,10,14-trimethyl-2-pentadecanone (Ahmadzadeh Sani et al., 2015 ▶; Kamali et al., 2014 ▶; Javidnia et al., 2010 ▶). α-Pinene and 6,1,14-trimethyl-2-pentadecanone were also reported as major components of the essential oil in another study (Akhlaghi et al., 2009 ▶).
Deregulation of apoptosis in cancer cells has been proposed as the underling mechanism of tumor growth and invasion. Agents interfere with enzymes and proteins which contribute to apoptosis cascade are widely investigated as chemotherapeutics. Apoptosis-enhancing plants and chemicals are classified as putative cancer preventive or therapeutics (Tayarani-Najaran et al., 2014 ▶). Once the cytotoxic activity of B. multifidi verified in AlamarBlue® assay, the plant was investigated for potential apoptotic activity. Although the cytotoxic activity of the plant was higher in HEK293 cells, but this is preliminary study and further studies are recommended to compare its malignant and normal cell toxicity. PI staining of the cells treated with B. multifidi showed the apoptotic activity of the plant. B. multifidi increased the sub G1 peak of treated cells and can be introduced as an apoptosis-enhancing plant. However, further mechanistic investigation will reveal the exact underling mechanisms. Analytical studies to investigate the phytochemicals responsible for biological activity of the plant should also be done in future. In conclusion, although the plant did not show selective cytotoxic activity on cancer cells but it merits further investigation for elucidation of its bioactive cytotoxic compounds.
Acknowledgements
This work was supported by grants from North Khorasan University of Medical Sciences and Research Affairs of Mashhad University of Medical Sciences.
Conflict of interest
There is no conflict of interests.
References
- Ahmadzadeh Sani T, Golmakani E, Mohammadi A, Feyzi P, Kamali H. Optimization of pressurized hot water extraction on the extract yield and antioxidant activity from Biebersteinia multifida DC using a modified supercritical fluid extractor. J Supercrit Fluids. 2014;94:130–137. [Google Scholar]
- Akhlaghi H, Shafaghat A, Mohammadhosseini M. Chemical composition of the essential oil from leaves of Biebersteinia multifida DC. growing wild in Iran. J Essent Oil Bear Pl. 2009;12:365–368. [Google Scholar]
- Arifkhodzhaev AO, Arifkhodzhaev KA, Kondratenko ES. Polysaccharides of saponin-bearing plants. II. Isolation and characterization of the polysaccharides of Biebersteinia multifidi. Chem Nat Compd. 1985;21:714–716. [Google Scholar]
- Arifkhodzhaev AO, Rakhimov DA. Polysaccharides of saponin-bearing plants. IV. Structure of glucans A, B, and C of Biebersteinia multifidi. Chem Nat Compd. 1993;29:151–153. [Google Scholar]
- Arifkhodzhaev AO, Rakhimov DA. Polysaccharides of saponin-bearing plants. V. Structural investigation of glucans A, B, and C and their oligosaccharides from Biebersteinia multifida plants. Chem Nat Compd. 1994;30:655–660. [Google Scholar]
- Boozari M, Mohammadi A, Asili A, Emami SA, Tayarani-Najaran Z. Growth inhibition and apoptosis induction by Scutellaria pinnatifida A Ham on HL-60 and K562 leukemic cell lines. Inviron Toxicol Pharmacol. 2015;39:307–312. doi: 10.1016/j.etap.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Efferth T, Herrmann F, Tahrani A, Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine. 2011;18:959–69. doi: 10.1016/j.phymed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Ericsson T, Blank A, von Hagens C, Ashton M, Äbelö A. Population pharmacokinetics of artesunate and dihydroartemisinin during long-term oral administration of artesunate to patients with metastatic breast cancer. Eur J ClinPharmacol. 2014;70:1453–63. doi: 10.1007/s00228-014-1754-2. [DOI] [PubMed] [Google Scholar]
- Farsam H, Amanlou M, Dehpour AR, Jahaniani F. Antiinflammatory and analgesic activity of Biebersteinia multifida DC root extract. J Ethnopharmacol. 2000;17:443–447. doi: 10.1016/s0378-8741(00)00174-4. [DOI] [PubMed] [Google Scholar]
- Fulda S. Modulation of apoptosis by natural products for cancer therapy. Planta Med. 2010;76:1075–1079. doi: 10.1055/s-0030-1249961. [DOI] [PubMed] [Google Scholar]
- Greenham J, Vassiliades DD, Harborne JB, Williams CA, Eagles J, Grayer RJ, Veitch NC. A distinctive flavonoid chemistry for the anomalous genus Biebersteinia. Phytochemistry. 2001;56:87–91. doi: 10.1016/s0031-9422(00)00355-1. [DOI] [PubMed] [Google Scholar]
- Hashem Dabaghian F, Entezari M, Ghobadi A, Hashemi M. Antimutagenicity and Anticancer Effects of Biebersteinia multifida DC. Annu Res Rev Biol. 2014;4(6):906–913. [Google Scholar]
- Janighorban M. Geraniaceae. In: Assadi M, editor. Flora of Iran. Tehran: Research Institute of Forests and Ragelands, No. 62; 2009. pp. 120–124. [Google Scholar]
- Jansen FH, Adoubi I, J C KC, DE Cnodder T, Jansen N, Tschulakow A, Efferth T. First study of oral Artenimol-R in advanced cervical cancer: clinical benefit, tolerability and tumor markers. Anticancer Res. 2011;31:4417–22. [PubMed] [Google Scholar]
- Javidnia K, Miri R, Soltani M, Khosravi AR. Essential oil composition of Biebersteinia multifida DC (Biebersteiniaceae) from Iran. J Essent Oil Res. 2010;22:611–612. [Google Scholar]
- Kamali H, Golmakani E, Golshan A, Mohammadi A, Sani TA. Optimization of ethanol modified supercritical carbon dioxide on the extract yield and antioxidant activity from Biebersteinia multifida DC. J Supercrit Fluid. 2014;91:46–52. [Google Scholar]
- Khakpour S, Akhlaghdoust M, Naimi S, Mirlohi MJ, Abedian M, Seyed-Forootan NS, Foroughi F. Effect of Biebersteinia multifida DC Root Extract on Cholesterol in Mice. Zahedan J Res Med Sci. 2013;15:49–51. [Google Scholar]
- Kurbanov D, Zharekeev KB. Study of alkaloids of Biebersteinia multifida and Peganumharmala from Karakalpakia (Russian) Chem Nat Compd. 1974;10:715–716. [Google Scholar]
- Monsef-Esfahani HR, Amini M, Goodarzi N, Saiedmohammadi F, Hajiaghaee R, Faramarzi MA, Tofighi Z, Ghahremani MH. Coumarin compounds of Biebersteinia multifida roots show potential anxiolytic effects in mice. Daru. 2013;21:51. doi: 10.1186/2008-2231-21-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaez M, Emami SA, Tayarani-Najjaran Z. Growth inhibition and apoptosis induction of Scutellaria luteo-coerulea Bornm & Sint on leukemia cancer cell lines K562 and HL-60. Avicenna J Phytomed. 2015;5:553–559. [PMC free article] [PubMed] [Google Scholar]
- Mousavi SH, Davari AS, Iranshahi M, Sabouri-Rad S, Tayarani-Najaran Z. Comparative analysis of the cytotoxic effect of 7-prenyloxycoumarin compounds and herniarin on MCF-7 cell line. Avicenna J Phytomed. 2015;5:520–530. [PMC free article] [PubMed] [Google Scholar]
- Muellner AN. "Biebersteiniaceae". In: Klaus Kubitzki., editor. The Families and Genera of Vascular Plants Vol. X: Flowering Plants Eudicots. Springer Verlag Berlin; 2011. 72 pp. ISBN 978-3-642-14396-0. [Google Scholar]
- Nicoletti I , Migliorati G , Pagliacci MC , Grignani F , Riccardi C A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Omurkamzinova VB, Maurel ND, Bikbulatova TN. Flavonoids of Biebersteinia multifida. Chem Nat Compd. 1991;27:636–637. [Google Scholar]
- Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–7. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahranavard S, Naghibi F, Mosaddegh M, Esmaeili S, Sarkhail P, Taghvaei M, Ghafari S. Cytotoxic activities of selected medicinal plants from Iran and phytochemical evaluation of the most potent extract. Res Pharm Sci. 2009;4:133–137. [PMC free article] [PubMed] [Google Scholar]
- Tayarani-Najaran Z, Amiri A, Karimi G, Emami SA, Asili J, Mousavi SH. Comparative studies of cytotoxic and apoptotic properties of different extracts and the essential oil of lavandula angustifolia on malignant and normal cells. Nutr Cancer. 2014;66:424–434. doi: 10.1080/01635581.2013.878736. [DOI] [PubMed] [Google Scholar]
- Taylor P, Colman L, Bajoon J. The search for plants with anticancer activity: pitfalls at the early stages. J Ethnopharmacol. 2014;158 Pt A:246–54. doi: 10.1016/j.jep.2014.10.034. [DOI] [PubMed] [Google Scholar]