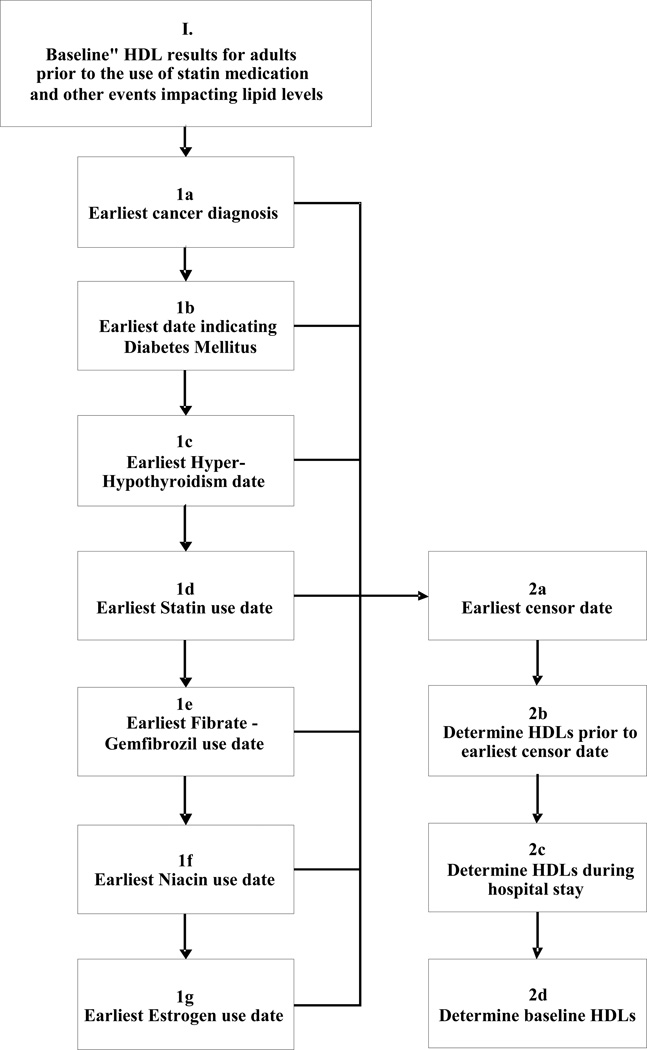
Figure 1.
Electronic characterization of baseline HDL. (1) All fasting lipid panels were extracted from the electronic medical record of each PMRP study participant. (1a – 1g) The record for each participant was interrogated identify the first date at which each subjects developed a comorbidity (or medication exposure) that would be expected to alter HDL cholesterol level: (1a) earliest cancer diagnosis in tumor registry; (1b) earliest date for diabetes mellitus based on an algorithm previously published in Wilke et al, 200723; (1c) earliest date of diagnosis for Hyperthyroidism or Hypothyroidism date (diagnostic codes 252.0–252.1, 253.0,253.9, prior to 1969 and 242.00–242.33, 242.90–242.93, 1969 to present) or exposure to levothyroxine, or TSH level outside normal range; (1d) earliest use of a commercially available statin; (1e) earliest use of gemfibrozil or fenofibrate; (1f) earliest use of niacin (restricted to prescription strength doses); (1g) earliest use non-contraceptive estrogen. Right panel: (2a–2b) Each individual’s HDL data string was censored according to diagnostic code and medication. (2c) Any datapoints obtained during hospitalization were filtered. (2d) All remaining data points were then used to model baseline HDL.