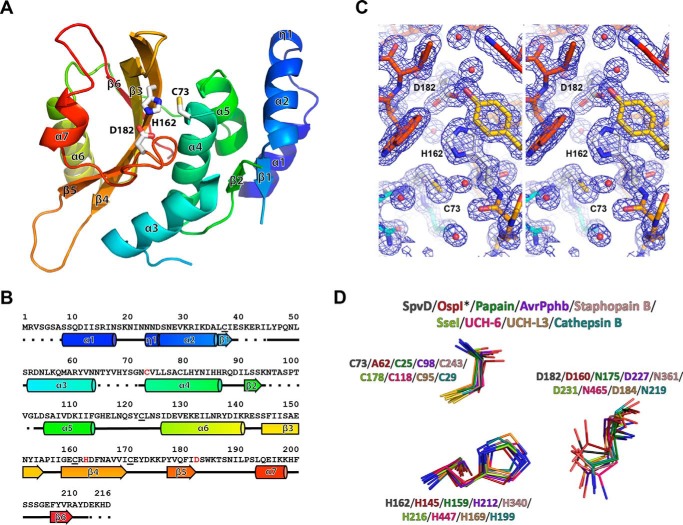
FIGURE 1.
SpvD adopted a papain-like fold with Cys-73, His-162, and Asp-182 catalytic triad. A, crystal structure of S. typhimurium SpvD4CS colored starting from N terminus (blue) and ending at C terminus (red). Secondary structure elements and catalytic triad (sticks) are labeled in black. Cys-73/His-162/Asp-182 catalytic triad is shown in sticks. The image was generated using PyMOL software. B, secondary structure elements mapped according to their respective amino acid sequence and colored as in A. In the sequence, the catalytic triad residues are colored in red. Surface cysteines mutated to serines are underlined. C, walleye stereo view of the 2Fo − Fc electron density map of the active site region of the SpvD structure. D, superimposition of catalytic triad components of SpvD, OspIC62A (PDB code 3W31), papain (PDB code 1PPN), AvrPphB (PDB code 1UKF), staphopain B (PDB code 1Y4H), SseI (PDB code 4G29), UCH-6 (PDB code 1VJV), UCH-L3 (PDB code 1XD3), and cathepsin B (PDB code 3AI8).