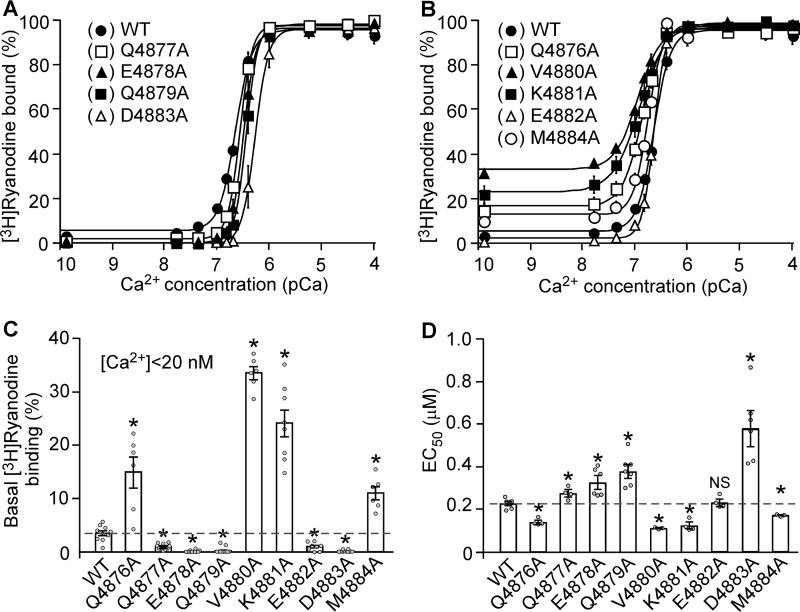
FIGURE 3.
Effect of the S6 inner helix mutations on [3H]ryanodine binding to RyR2. A and B, [3H]Ryanodine binding to cell lysate prepared from HEK293 cells expressing RyR2 WT and the S6 mutants Q4877A, E4878A, Q4879A, E4882A, and D4883A (A) or the S6 mutants Q4876A, V4880A, K4881A, and M4884A (B) was carried out at various Ca2+ concentrations (∼0.2 nm to 0.1 mm), 800 mm KCl, and 5 nm [3H]ryanodine. The amounts of [3H]ryanodine binding at various Ca2+ concentrations were normalized to the maximal binding (100%). C, mutating the cytoplasmic region of the S6 inner helix of RyR2 affects the basal level of [3H]ryanodine binding to RyR2 in the near absence of activating Ca2+ (<20 nm). D, the EC50 values of Ca2+ activation of [3H]ryanodine binding to RyR2 WT and the S6 mutants. The data points shown are mean ± S.E. from three to five separate experiments (*p < 0.05 versus WT).