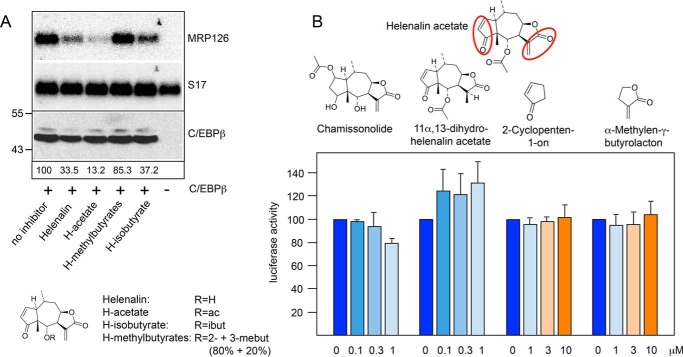
FIGURE 3.
Inhibitory activities of different sesquiterpene lactones. A, QT6 cells were transfected with expression vector for chicken C/EBPβ and treated with 1 μm of the indicated helenalin esters or left untreated, as indicated below the lanes. The last lane shows untransfected cells, which serve as control. Cells were harvested after 12 h and analyzed by Northern blotting for expression of MRP126 and S17 mRNAs (top and middle panel) and by Western blotting for the expression of C/EBPβ (bottom panel). Numbers below the lanes indicate the relative amounts of MRP126 mRNA determined by quantification with a phosphor image analyzer. ac, acetate; ibut, isobutyrate; mebut, methylbutyrate. B, the structure of helenalin acetate is shown at the top. Reactive α,β-unsaturated carbonyl groups are marked with red circles. The structures of chamissonolide, 11α,13-dihydrohelenalin acetate, 2-cyclopenten-1-one and α-methylene-γ-butyrolactone are shown below. Reporter assays were performed with QT6 fibroblasts transfected with the C/EBP-dependent luciferase reporter gene p-240luc, expression vector for chicken C/EBPβ and pCMVβ. Cells were cultivated for 12 h with the indicated concentrations of the compounds shown above and analyzed for luciferase and β-galactosidase activities as in Fig. 1. Error bars show the S.D.