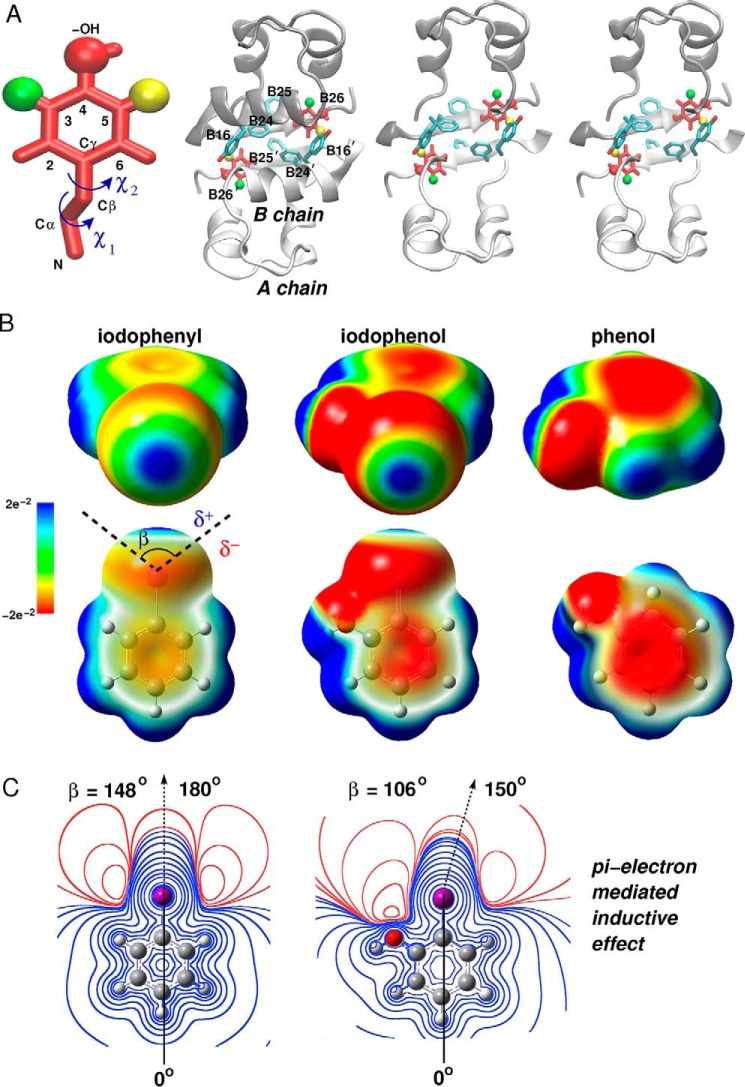
FIGURE 2.
Quantum chemistry of iodo-aromatic system. A, Tyr side chain with iodine in its 3- or 5-ring position (green and yellow, in and out, respectively) with carbon-atom positions labeled. Rotation angles for rigid-body modeling involve rotations around the Cα–Cβ bond (χ1) and Cβ–Cγ bond (χ2). The insulin dimer with B26 side chains (red) and side chains of TyrB16, PheB24, PheB25, and their dimer-related mates (blue). The right-hand side provides a cross-eyed stereo view of the modified dimer with the B chain helix removed for clarity. B, electrostatic potential (ESP) surface maps of phenol, iodophenol, and iodophenyl at the 0.001 e bohr−3 isodensity. The color scale of the surface potential ranges from −2.12 e−2 (red) through 0 (green) to 2.12 e−2 (blue). In the upper row the iodine (facing the viewer) exhibits the effect of the electron-donating –OH on the σ-hole. The lower row shows effects of iodine on the π-system of the phenol ring. In the 1st row the surface is opaque, and in the 2nd row the surface is transparent. Angle β represents the σ-hole size as delimited by black dashed lines. δ+ and δ− represent respective regions of positive and negative charge around the iodine. C, ESP contours of iodophenyl (left) and 2-iodophenol (right), at different isovalues, calculated in the plane of the aromatic ring. The halogen boundary represents a region of an electron isodensity of 10−3 e bohr−3 (111). Isocontours in the left and right panels are at the same heights but in uneven separations. The σ-hole size, defined by an angle β (B), was calculated from the angular profile of the ESP on the intersection line of the 2D grid and halogen boundary where the ESP changes its sign (55); positive ESP and negative ESP regions are shown in blue and red, respectively. The black dashed arrow indicates directionality of the C–I bond.