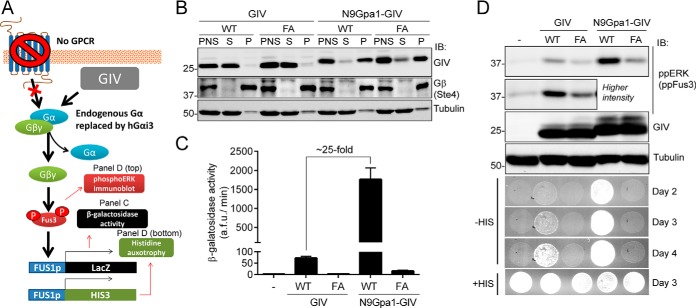
FIGURE 2.
Membrane association of GIV enhances its ability to activate G protein signaling in yeast. A, schematic diagram of the yeast-based assays used to monitor G protein signaling. A genetically engineered strain lacking endogenous GPCRs and with human Gαi3 replacing the endogenous Gα Gpa1 was used to determine the levels of G protein activation upon expression of exogenous GIV. G protein activation was determined by immunoblotting (IB) for Fus3 phosphorylation or two gene reporters controlled by the Fus1 promoter (β-galactosidase activity or histidine auxotrophy). B, yeast cells expressing unmodified GIV (aa 1660–1870) or fused to a membrane-targeting sequence corresponding to the first 9 aa of Gpa1 (N9Gpa1-GIV) were homogenized in the absence of detergents, and the PNS was separated into supernatant (S) and pellet (P) fractions after 100,000 × g centrifugation. Immunoblots of equal aliquots of each fraction from one experiment representative of four are shown. C, membrane-targeted N9Gpa1-GIV enhances G protein-dependent β-galactosidase activity ∼25-fold more than cytosolic GIV. The results are the averages of n = 4, and error bars are the S.E. D, membrane-targeted N9Gpa1-GIV induces more Fus3 phosphorylation (ppERK/ppFus3; top, immunoblot panels) and more rapid cell growth in media lacking histidine (bottom, spot growth panels) than cytosolic GIV. One experiment representative of four is shown.