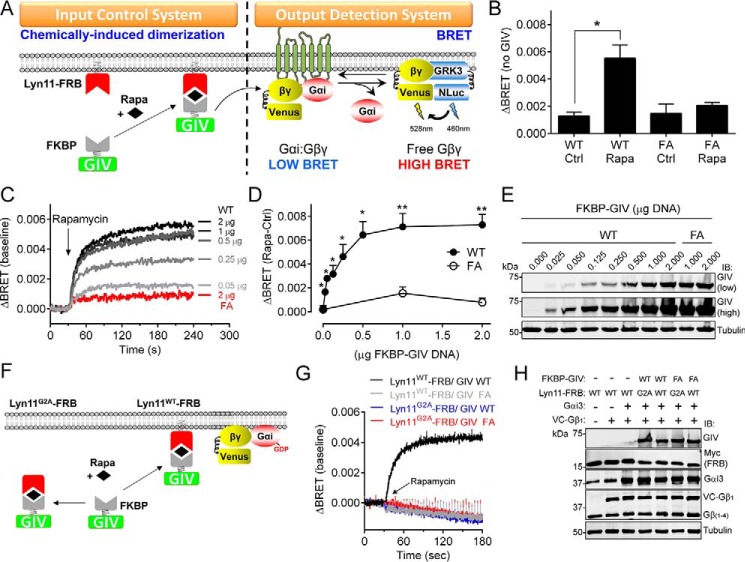
FIGURE 3.
Acute translocation of GIV to membranes induces rapid G protein activation in mammalian cells. A, translocation of GIV to membranes is controlled by CID using the FKBP-rapamycin-FRB system. FKBP-fused GIV is recruited to membranes upon rapamycin-induced binding of FKBP to the FRB domain that is fused to a membrane-targeting sequence (Lyn11). G protein activation is determined by BRET. Dissociation of Gαi3:Gβγ trimers upon G protein activation leads to the release of Venus-tagged Gβγ (V-Gβγ), which binds to the C-terminal domain of GRK3 fused to nanoluciferase (GRK3-Nluc) and causes an increase of BRET signal. B, rapamycin-induced translocation of FKBP-GIV (aa 1660–1870, 0.25 μg of plasmid DNA) WT but not the GEF-deficient F1685A (FA, 1 μg of plasmid DNA) mutant leads to G protein-dependent BRET. HEK293T cells expressing all the required assay components were treated with rapamycin (0.5 μm, Rapa) or ethanol (0.005%, Ctrl) for 2 min before BRET measurements. The results are presented as the increases in BRET (ΔBRET) compared with cells not expressing GIV (average of n = 3). The error bars are the S.E. *, p < 0.05 using Student's t test. C–E, dose-dependent G protein activation induced by membrane recruitment of GIV. Representative traces of kinetic BRET measurements of cells transfected with the indicated amounts of FKBP-GIV plasmids and stimulated with rapamycin are shown in C. Quantification of the average increase in BRET (ΔBRET) after 2 min of stimulation with rapamycin (0.5 μm, Rapa) compared with ethanol (0.05%, Ctrl) from five independent experiments is shown in D. The error bars are the S.E. *, p < 0.05; **, p < 0.01 using Student's t test. Representative immunoblots of the cells used in these experiments are shown in E. F–H, membrane targeting of FRB is required FKBP-GIV-mediated G protein activation upon rapamycin stimulation. Mutation of the myristoylation site in the Lyn11 sequence (Lyn11G2A) is predicted to preclude rapamycin-induced translocation of FKBP-GIV and subsequent G protein activation (F). Kinetic BRET measurements of cells transfected with the indicated Lyn11-FRB and FKBP-GIV (2 μg) constructs and stimulated with rapamycin at the indicated time (arrow) are shown in G, and representative immunoblots of the cells used in these experiments are shown in H. The results are the average of three independent experiments, and the error bars are the S.E. (shown only at 5-s intervals for clarity).