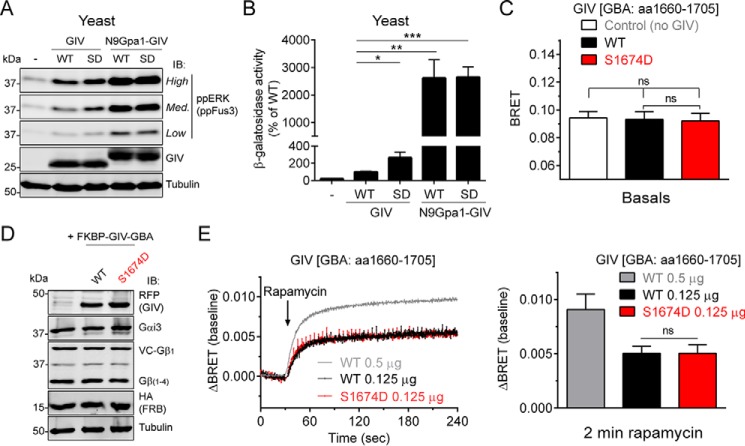
FIGURE 6.
Membrane recruitment of GIV is a mechanism of G protein activation more potent than its phosphorylation at Ser1674. A and B, membrane targeting of GIV enhances G protein activation in yeast much more potently than the phosphomimicking S1674D mutation and S1674D fails to further enhance G protein activation induced by membrane-anchored GIV. G protein activation in yeast was determined by ppFus3 immunoblotting (A, IB) or β-galactosidase reporter activity (B) in the strain described for Fig. 2 expressing cytoplasmic (GIV) or membrane-anchored (N9Gpa1-GIV) versions of GIV. SD, phosphomimicking S1674D mutant. One experiment representative of four is shown for the immunoblotting results (A) and the average of n = 4–5 for the β-galactosidase results (B). C–E, expression of FKBP-GIV-GBA S1674D at the same concentration as WT (D) does not affect basal (C) or GIV-stimulated (E) G protein activity as determined by BRET in mammalian cells. HEK293T cells were transfected with plasmids for all the components required for CID (0.125 μg of FKBP-GIV-GBA; aa 1660–1705) and BRET-based G protein activity measurements as described for Fig. 3. BRET results (C and E) are the averages of three independent experiments, and the error bars are the S.E. The data corresponding to FKBP-GIV-GBA WT 0.5 μg from Fig. 5 is presented here for comparison. The bar graph in E corresponds to the ΔBRET values 2 min after rapamycin addition. Representative immunoblots of the cells used in these experiments are shown in D. The error bars represent the S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant using Student's t test.